Journal of Photochemistry and Photobiology A: Chemistry ( IF 4.1 ) Pub Date : 2020-12-17 , DOI: 10.1016/j.jphotochem.2020.113102 Mohamed Larbi Djaballah , Slimane Merouani , Hafida Bendjama , Oualid Hamdaoui
|
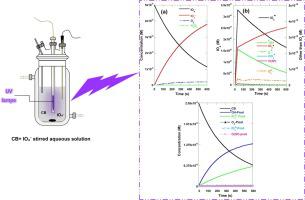
This paper presents the first modeling study on UV/periodate (IO4–) advanced oxidation process. A reaction mechanism consisting in 45 chemical reactions including a number of radicals (IO3•, IO4•, •OH, O•–, HO2•, O2•–, O3•–) and non-radical intermediates/products (O(3 P), O3, IO3–, H2O2, HO2–, I2O6 and I2O8) has been developed to assess the reactive species generation, use and distribution during the oxidation of one model azo dye, chlorazol black (CB), by the periodate photoactivated process. The model fitted excellently the CB degradation data over a wide range of solution pH and initial IO4– concentration. Unavailable second-order rate constants of several important reactions were optimized using the genetic algorithm. Those include O3 + IO3• → IO4• + O2 (k = 1 × 108 M−1s−1), 2IO4• → I2O8 (k = 4.68 × 108 M−1s−1), reaction of CB with •OH (k = 1 × 109 M−1s−1), IO3• (k = 2.63 × 108 M−1s−1), IO4• (k = 0 M−1s−1), O(3 P) (k = 0 M−1s−1) and O3 (k = 0.574 M−1s−1). Besides, the first order kinetic constant of periodate photolysis, IO4– + hv → IO3• + O•– and IO4– + hv → IO3– + O(3 P), were respectively (0.5–4.16)×10-3 s−1 and ∼2 × 10-4 s−1, indicating that the radical pathway for IO4– photolysis is predominately the IO3–-releasing path.  OH and IO3
OH and IO3 were found to play the key role in the CB degradation. The concentration of radicals increased with pH decrease and initial periodate concentration rise, favoring higher degradation rate at acidic conditions and higher IO4– dosages. The selectivity analysis showed that the contribution of these species is ∼ 79 % for •OH and ∼ 21 % for IO3•, under various conditions of pH and [IO4–]0. This study would helpfully provide some practical indications for the application of UV/periodate AOP
were found to play the key role in the CB degradation. The concentration of radicals increased with pH decrease and initial periodate concentration rise, favoring higher degradation rate at acidic conditions and higher IO4– dosages. The selectivity analysis showed that the contribution of these species is ∼ 79 % for •OH and ∼ 21 % for IO3•, under various conditions of pH and [IO4–]0. This study would helpfully provide some practical indications for the application of UV/periodate AOP
中文翻译:

基于高碘酸盐光活化过程的基于自由基的动力学模型用于水溶液中氯唑黑氧化降解的建立
本文介绍了有关UV /高碘酸盐(IO 4 –)高级氧化过程的第一个建模研究。由45种化学反应组成的反应机理,其中包括许多自由基(IO 3 •,IO 4 •,• OH,O •–,HO 2 •,O 2 •–,O 3 •–)和非自由基中间体/产物(O(3 P),O 3,IO 3 –,H 2 O 2,HO 2 –,I 2 O 6和I 2 O8)已被开发出来,以评估高碘酸盐光活化过程在一种模型偶氮染料氯唑黑(CB)的氧化过程中反应性物质的产生,使用和分布。该模型非常适合在溶液pH和初始IO 4 –浓度范围内的CB降解数据。使用遗传算法优化了几个重要反应的不可用二级速率常数。包括O 3 + IO 3 • →IO 4 • + O 2(k = 1×10 8 M -1 s -1),2IO 4 • →I 2 O 8(k = 4.68×10 8 M -1 s -1),CB与• OH(k = 1×10 9 M -1 s -1),IO 3 •(k = 2.63×10 8 M -1 s)-1),IO 4 •(k = 0 M -1 s -1),O(3 P)(k = 0 M -1 s -1)和O 3(k = 0.574 M -1 s)-1)。此外,高碘酸盐光解的一阶动力学常数IO 4 – + hv →IO 3 • + O •–和IO 4 – + hv →IO 3 – + O(3 P)分别为(0.5–4.16)×10 -3 š -1和〜2×10 -4小号-1,这表明对于IO自由基途径4 -光解是主要在IO 3 - -释放路径。 OH和IO 3
OH和IO 3 被发现在炭黑降解中起关键作用。自由基的浓度随pH的降低和高碘酸盐的初始浓度的增加而增加,有利于在酸性条件下更高的降解速率和更高的IO 4 –剂量。选择性分析表明,这些物质的贡献是〜79%为• OH和〜21%为IO 3 •,pH值的各种条件和[IO下4 - ] 0。该研究将为紫外线/高碘酸盐AOP的应用提供一些实用的指示。
被发现在炭黑降解中起关键作用。自由基的浓度随pH的降低和高碘酸盐的初始浓度的增加而增加,有利于在酸性条件下更高的降解速率和更高的IO 4 –剂量。选择性分析表明,这些物质的贡献是〜79%为• OH和〜21%为IO 3 •,pH值的各种条件和[IO下4 - ] 0。该研究将为紫外线/高碘酸盐AOP的应用提供一些实用的指示。











































 京公网安备 11010802027423号
京公网安备 11010802027423号