当前位置:
X-MOL 学术
›
J. Inherit. Metab. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combined proteomic and lipidomic studies in Pompe disease allow a better disease mechanism understanding
Journal of Inherited Metabolic Disease ( IF 4.2 ) Pub Date : 2020-12-15 , DOI: 10.1002/jimd.12344 Anna Sidorina 1 , Giulio Catesini 1 , Stefano Levi Mortera 2 , Valeria Marzano 2 , Lorenza Putignani 2, 3 , Sara Boenzi 1 , Roberta Taurisano 1 , Matteo Garibaldi 4 , Federica Deodato 1 , Carlo Dionisi-Vici 1
Journal of Inherited Metabolic Disease ( IF 4.2 ) Pub Date : 2020-12-15 , DOI: 10.1002/jimd.12344 Anna Sidorina 1 , Giulio Catesini 1 , Stefano Levi Mortera 2 , Valeria Marzano 2 , Lorenza Putignani 2, 3 , Sara Boenzi 1 , Roberta Taurisano 1 , Matteo Garibaldi 4 , Federica Deodato 1 , Carlo Dionisi-Vici 1
Affiliation
|
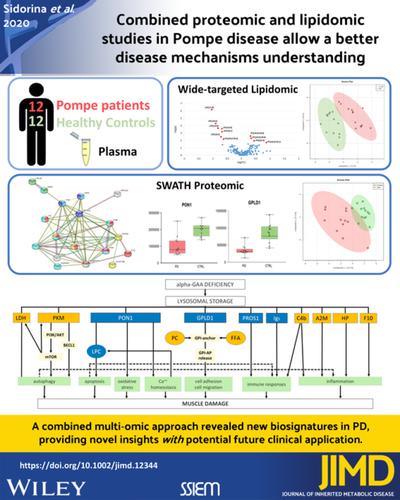
Pompe disease (PD) is caused by deficiency of the enzyme acid α‐glucosidase resulting in glycogen accumulation in lysosomes. Clinical symptoms include skeletal myopathy, respiratory failure, and cardiac hypertrophy. We studied plasma proteomic and lipidomic profiles in 12 PD patients compared to age‐matched controls. The proteomic profiles were analyzed by nLC‐MS/MS SWATH method. Wide‐targeted lipidomic analysis was performed by LC‐IMS/MS, allowing to quantify >1100 lipid species, spanning 13 classes. Significant differences were found for 16 proteins, with four showing the most relevant changes (GPLD1, PON1, LDHB, PKM). Lipidomic analysis showed elevated levels of three phosphatidylcholines and of the free fatty acid 22:4, and reduced levels of six lysophosphatidylcholines. Up‐regulated glycolytic enzymes (LDHB and PKM) are involved in autophagy and glycogen metabolism, while down‐regulated PON1 and GPLD1 combined with lipidomic data indicate an abnormal phospholipid metabolism. Reduced GPLD1 and dysregulation of lipids with acyl‐chains characteristic of GPI‐anchor structure suggest the potential involvement of GPI‐anchor system in PD. Results of proteomic analysis displayed the involvement of multiple cellular functions affecting inflammatory, immune and antioxidant responses, autophagy, Ca2+‐homeostasis, and cell adhesion. The combined multi‐omic approach revealed new biosignatures in PD, providing novel insights in disease pathophysiology with potential future clinical application.
中文翻译:

庞贝病的蛋白质组学和脂质组学联合研究可以更好地了解疾病机制
庞贝病 (PD) 是由酸性α-葡萄糖苷酶缺乏导致糖原在溶酶体中积累引起的。临床症状包括骨骼肌病、呼吸衰竭和心脏肥大。与年龄匹配的对照相比,我们研究了 12 名 PD 患者的血浆蛋白质组学和脂质组学特征。通过 nLC-MS/MS SWATH 方法分析蛋白质组学特征。LC-IMS/MS 进行了广泛靶向的脂质组学分析,可以量化超过 1100 种脂质,跨越 13 个类别。发现了 16 种蛋白质的显着差异,其中四种显示出最相关的变化(GPLD1、PON1、LDHB、PKM)。脂质组学分析显示 3 种磷脂酰胆碱和 22:4 游离脂肪酸水平升高,而 6 种溶血磷脂酰胆碱水平降低。上调的糖酵解酶(LDHB 和 PKM)参与自噬和糖原代谢,而下调的 PON1 和 GPLD1 结合脂质组学数据表明磷脂代谢异常。降低的 GPLD1 和具有 GPI 锚结构特征的酰基链的脂质失调表明 GPI 锚系统可能参与 PD。蛋白质组学分析结果显示,多种细胞功能参与影响炎症、免疫和抗氧化反应、自噬、Ca2+-稳态和细胞粘附。组合的多组学方法揭示了 PD 的新生物特征,为疾病病理生理学提供了新的见解,并具有潜在的未来临床应用。
更新日期:2020-12-15
中文翻译:

庞贝病的蛋白质组学和脂质组学联合研究可以更好地了解疾病机制
庞贝病 (PD) 是由酸性α-葡萄糖苷酶缺乏导致糖原在溶酶体中积累引起的。临床症状包括骨骼肌病、呼吸衰竭和心脏肥大。与年龄匹配的对照相比,我们研究了 12 名 PD 患者的血浆蛋白质组学和脂质组学特征。通过 nLC-MS/MS SWATH 方法分析蛋白质组学特征。LC-IMS/MS 进行了广泛靶向的脂质组学分析,可以量化超过 1100 种脂质,跨越 13 个类别。发现了 16 种蛋白质的显着差异,其中四种显示出最相关的变化(GPLD1、PON1、LDHB、PKM)。脂质组学分析显示 3 种磷脂酰胆碱和 22:4 游离脂肪酸水平升高,而 6 种溶血磷脂酰胆碱水平降低。上调的糖酵解酶(LDHB 和 PKM)参与自噬和糖原代谢,而下调的 PON1 和 GPLD1 结合脂质组学数据表明磷脂代谢异常。降低的 GPLD1 和具有 GPI 锚结构特征的酰基链的脂质失调表明 GPI 锚系统可能参与 PD。蛋白质组学分析结果显示,多种细胞功能参与影响炎症、免疫和抗氧化反应、自噬、Ca2+-稳态和细胞粘附。组合的多组学方法揭示了 PD 的新生物特征,为疾病病理生理学提供了新的见解,并具有潜在的未来临床应用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号