Journal of Neuroradiology ( IF 3.0 ) Pub Date : 2020-12-16 , DOI: 10.1016/j.neurad.2020.11.003 Alejandro Tomasello 1 , David Hernandez 1 , Carlos Piñana 1 , Manuel Requena 2 , David S Liebeskind 3 , Raul G Nogueira 4 , Tudor Jovin 5 , Tommy Andersson 6 , Christoph Cognard 7 , Adnan Siddiqui 8 , Marc Ribo 2
|
Introduction
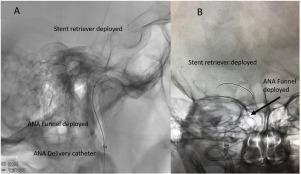
The ANA™ (Anaconda Biomed) thrombectomy system is a novel stroke thrombectomy device comprising a self-expanding funnel designed to reduce clot fragmentation by locally restricting flow while becoming as wide as the lodging artery. Once deployed, ANA allows distal aspiration in combination with a stentretriever (SR) to mobilize the clot into the funnel where it remains copped during extraction. We investigate safety and efficacy of ANA™ in a first-in-man study.
Methods
Prospective data was collected on 35 consecutive patients treated as first line with ANA™ at a single centre. Outcome measures included per-pass reperfusion scores, symptomatic intracerebral hemorrhage (sICH), NIHSS at day 5, and mRS at 90 days.
Results
Median NIHSS was 12(9−18). Sites of primary occlusion were: 5 ICA, 15 M1-MCA, 15 M2-MCA. Primary performance endpoint, mTICI 2b-3 within 3 passes without rescue therapy was achieved in 91.4% (n = 32) of patients; rate of complete recanalization (mTICI 2c-3) was 65.7%. First pass complete recanalization rate was 42.9%, and median number of ANA passes 1(IQR: 1−2). In 17.1% (n = 6) rescue treatment was used; median number of rescue passes was 2(1–7), leading to a final mTICI2b-3rate of 94.3% (n = 33). There were no device related serious adverse events, and rate of sICH was 5.7% (n = 2). At 5 days median NIHSS was 1 (IQR 1−6) and 90 days mRS 0−2 was achieved in 60% of patients.
Conclusions
In this initial clinical experience, the ANA™ device achieved a high rate of complete recanalization with a good safety profile and favourable 90 days clinical outcomes.
中文翻译:

采用新型装置进行机械取栓:ANA 取栓装置的初步临床经验
介绍
ANA™(Anaconda Biomed)血栓切除术系统是一种新型中风血栓切除术装置,包括一个自膨胀漏斗,旨在通过局部限制流动来减少凝块碎片,同时变得与寄宿动脉一样宽。一旦部署,ANA 允许远端抽吸与 stentretriever (SR) 相结合,以将凝块移动到漏斗中,并在提取过程中保持堵塞。我们在一项首次人体研究中调查 ANA™ 的安全性和有效性。
方法
在一个中心收集了 35 名连续接受 ANA™ 一线治疗的患者的前瞻性数据。结果测量包括每次再灌注评分、症状性脑出血 (sICH)、第 5 天的 NIHSS 和第 90 天的 mRS。
结果
NIHSS 中位数为 12(9-18)。主要闭塞部位为:5 个 ICA、15 个 M1-MCA、15 个 M2-MCA。91.4% (n = 32) 的患者达到主要性能终点,3 次内 mTICI 2b-3 且未进行抢救治疗;完全再通率 (mTICI 2c-3) 为 65.7%。首次通过完全再通率为 42.9%,ANA 通过的中位数为 1(IQR:1-2)。在 17.1% (n = 6) 中使用了抢救治疗;救援通行证的中位数为 2(1-7),导致最终 mTICI2b-3 率为 94.3%(n = 33)。没有与设备相关的严重不良事件,sICH 发生率为 5.7%(n = 2)。5 天时,NIHSS 中位数为 1(IQR 1-6),90 天时,60% 的患者达到 mRS 0-2。
结论
在最初的临床经验中,ANA™ 装置实现了高完全再通率,具有良好的安全性和良好的 90 天临床结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号