Structure ( IF 4.4 ) Pub Date : 2020-12-15 , DOI: 10.1016/j.str.2020.11.016 Weidi Chen 1 , Zeyu Shen 2 , Sabrina Asteriti 3 , Zijing Chen 4 , Fei Ye 2 , Ziling Sun 5 , Jun Wan 6 , Craig Montell 4 , Roger C Hardie 7 , Wei Liu 5 , Mingjie Zhang 6
|
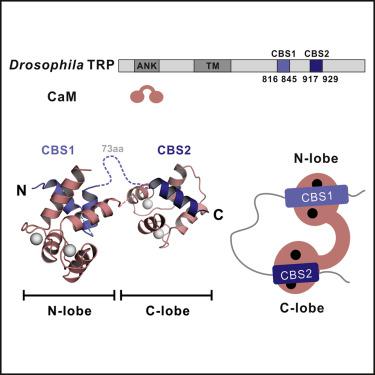
Drosophila TRP is a calcium-permeable cation channel essential for fly visual signal transduction. During phototransduction, Ca2+ mediates both positive and negative feedback regulation on TRP channel activity, possibly via binding to calmodulin (CaM). However, the molecular mechanism underlying Ca2+ modulated CaM/TRP interaction is poorly understood. Here, we discover an unexpected, Ca2+-dependent binding mode between CaM and TRP. The TRP tail contains two CaM binding sites (CBS1 and CBS2) separated by an ∼70-residue linker. CBS1 binds to the CaM N-lobe and CBS2 recognizes the CaM C-lobe. Structural studies reveal the lobe-specific binding of CaM to CBS1&2. Mutations introduced in both CBS1 and CBS2 eliminated CaM binding in full-length TRP, but surprisingly had no effect on the response to light under physiological conditions, suggesting alternative mechanisms governing Ca2+-mediated feedback on the channel activity. Finally, we discover that TRPC4, the closest mammalian paralog of Drosophila TRP, adopts a similar CaM binding mode.
中文翻译:

钙调蛋白以一种意想不到的方式与果蝇 TRP 结合
果蝇TRP 是一种钙可渗透的阳离子通道,对果蝇视觉信号转导至关重要。在光转导过程中,Ca 2+可能通过与钙调蛋白 (CaM) 结合来介导对 TRP 通道活性的正反馈和负反馈调节。然而,Ca 2+调节CaM/TRP 相互作用的分子机制知之甚少。在这里,我们发现了一个意想不到的 Ca 2+CaM 和 TRP 之间的依赖结合模式。TRP 尾部包含两个 CaM 结合位点(CBS1 和 CBS2),由一个~70 个残基的接头隔开。CBS1 与 CaM N 叶结合,CBS2 识别 CaM C 叶。结构研究揭示了 CaM 与 CBS1&2 的叶特异性结合。CBS1 和 CBS2 中引入的突变消除了全长 TRP 中的 CaM 结合,但令人惊讶的是对生理条件下对光的反应没有影响,这表明控制 Ca 2+介导的通道活性反馈的替代机制。最后,我们发现与果蝇TRP最接近的哺乳动物旁系同源物 TRPC4采用类似的 CaM 结合模式。











































 京公网安备 11010802027423号
京公网安备 11010802027423号