当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing Biomolecular Interactions by a Pattern-Forming Peptide–Conjugate Sensor
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-12-14 , DOI: 10.1021/acs.bioconjchem.0c00596 Tamara Heermann 1 , Henri G Franquelim 1 , Philipp Glock 1 , Leon Harrington 1 , Petra Schwille 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-12-14 , DOI: 10.1021/acs.bioconjchem.0c00596 Tamara Heermann 1 , Henri G Franquelim 1 , Philipp Glock 1 , Leon Harrington 1 , Petra Schwille 1
Affiliation
|
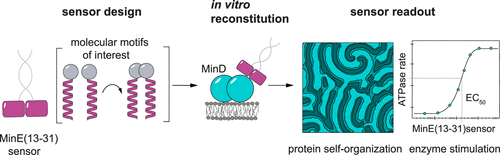
As a key mechanism underpinning many biological processes, protein self-organization has been extensively studied. However, the potential to apply the distinctive, nonlinear biochemical properties of such self-organizing systems to biotechnological problems such as the facile detection and characterization of biomolecular interactions has not yet been explored. Here, we describe an in vitro assay in a 96-well plate format that harnesses the emergent behavior of the Escherichia coli Min system to provide a readout of biomolecular interactions. Crucial for the development of our approach is a minimal MinE-derived peptide that stimulates MinD ATPase activity only when dimerized. We found that this behavior could be induced via any pair of foreign, mutually binding molecular entities fused to the minimal MinE peptide. The resulting MinD ATPase activity and the spatiotemporal nature of the produced protein patterns quantitatively correlate with the affinity of the fused binding partners, thereby enabling a highly sensitive assay for biomolecular interactions. Our assay thus provides a unique means of quantitatively visualizing biomolecular interactions and may prove useful for the assessment of domain interactions within protein libraries and for the facile investigation of potential inhibitors of protein–protein interactions.
中文翻译:

通过模式形成肽-缀合物传感器探测生物分子相互作用
作为支撑许多生物过程的关键机制,蛋白质自组织已被广泛研究。然而,尚未探索将这种自组织系统独特的非线性生化特性应用于生物技术问题(如生物分子相互作用的简易检测和表征)的潜力。在这里,我们描述了一种 96 孔板形式的体外测定,该测定利用了大肠杆菌的紧急行为Min 系统提供生物分子相互作用的读数。开发我们方法的关键是一种最小的 MinE 衍生肽,它仅在二聚化时刺激 MinD ATPase 活性。我们发现这种行为可以通过任何一对与最小 MinE 肽融合的外来、相互结合的分子实体来诱导。由此产生的 MinD ATPase 活性和产生的蛋白质模式的时空性质与融合结合伴侣的亲和力定量相关,从而能够对生物分子相互作用进行高度灵敏的测定。因此,我们的测定提供了一种独特的定量可视化生物分子相互作用的方法,并且可能证明对评估蛋白质文库中的域相互作用和轻松研究蛋白质-蛋白质相互作用的潜在抑制剂很有用。
更新日期:2021-01-20
中文翻译:

通过模式形成肽-缀合物传感器探测生物分子相互作用
作为支撑许多生物过程的关键机制,蛋白质自组织已被广泛研究。然而,尚未探索将这种自组织系统独特的非线性生化特性应用于生物技术问题(如生物分子相互作用的简易检测和表征)的潜力。在这里,我们描述了一种 96 孔板形式的体外测定,该测定利用了大肠杆菌的紧急行为Min 系统提供生物分子相互作用的读数。开发我们方法的关键是一种最小的 MinE 衍生肽,它仅在二聚化时刺激 MinD ATPase 活性。我们发现这种行为可以通过任何一对与最小 MinE 肽融合的外来、相互结合的分子实体来诱导。由此产生的 MinD ATPase 活性和产生的蛋白质模式的时空性质与融合结合伴侣的亲和力定量相关,从而能够对生物分子相互作用进行高度灵敏的测定。因此,我们的测定提供了一种独特的定量可视化生物分子相互作用的方法,并且可能证明对评估蛋白质文库中的域相互作用和轻松研究蛋白质-蛋白质相互作用的潜在抑制剂很有用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号