Cell Metabolism ( IF 29.0 ) Pub Date : 2020-12-14 , DOI: 10.1016/j.cmet.2020.11.020 Guo-Fang Zhang 1 , Mette V Jensen 2 , Sarah M Gray 2 , Kimberley El 2 , You Wang 2 , Danhong Lu 2 , Thomas C Becker 1 , Jonathan E Campbell 3 , Christopher B Newgard 3
|
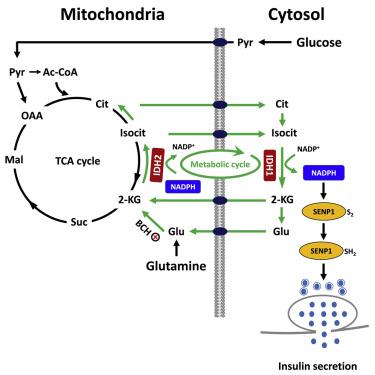
Metabolic fuels regulate insulin secretion by generating second messengers that drive insulin granule exocytosis, but the biochemical pathways involved are incompletely understood. Here we demonstrate that stimulation of rat insulinoma cells or primary rat islets with glucose or glutamine + 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (Gln + BCH) induces reductive, “counter-clockwise” tricarboxylic acid (TCA) cycle flux of glutamine to citrate. Molecular or pharmacologic suppression of isocitrate dehydrogenase-2 (IDH2), which catalyzes reductive carboxylation of 2-ketoglutarate to isocitrate, results in impairment of glucose- and Gln + BCH-stimulated reductive TCA cycle flux, lowering of NADPH levels, and inhibition of insulin secretion. Pharmacologic suppression of IDH2 also inhibits insulin secretion in living mice. Reductive TCA cycle flux has been proposed as a mechanism for generation of biomass in cancer cells. Here we demonstrate that reductive TCA cycle flux also produces stimulus-secretion coupling factors that regulate insulin secretion, including in non-dividing cells.
中文翻译:

还原性 TCA 循环代谢促进谷氨酰胺和葡萄糖刺激的胰岛素分泌
代谢燃料通过产生驱动胰岛素颗粒胞吐作用的第二信使来调节胰岛素分泌,但所涉及的生化途径尚不完全清楚。在这里,我们证明了用葡萄糖或谷氨酰胺 + 2-氨基双环-(2,2,1)-庚烷-2-羧酸 (Gln + BCH) 刺激大鼠胰岛素瘤细胞或原代大鼠胰岛诱导还原性“逆时针”三羧酸谷氨酰胺到柠檬酸盐的酸 (TCA) 循环通量。异柠檬酸脱氢酶 2 (IDH2) 的分子或药理学抑制,可催化 2-酮戊二酸还原羧化为异柠檬酸,导致葡萄糖和 Gln + BCH 刺激的还原性 TCA 循环通量受损、NADPH 水平降低和胰岛素抑制分泌。IDH2的药理学抑制也抑制活小鼠的胰岛素分泌。已提出还原性 TCA 循环通量作为在癌细胞中产生生物质的机制。在这里,我们证明还原性 TCA 循环通量也产生调节胰岛素分泌的刺激 - 分泌耦合因子,包括在非分裂细胞中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号