当前位置:
X-MOL 学术
›
Clin. Exp. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pre‐existing influenza‐specific nasal IgA or nasal viral infection does not affect live attenuated influenza vaccine immunogenicity in children
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-12-13 , DOI: 10.1111/cei.13564 M E Cole 1 , R Kundu 2, 3 , A F Abdulla 1 , N Andrews 4 , K Hoschler 4 , J Southern 4 , D Jackson 4 , E Miller 4 , M Zambon 4 , P J Turner 2, 3 , J S Tregoning 1, 2
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-12-13 , DOI: 10.1111/cei.13564 M E Cole 1 , R Kundu 2, 3 , A F Abdulla 1 , N Andrews 4 , K Hoschler 4 , J Southern 4 , D Jackson 4 , E Miller 4 , M Zambon 4 , P J Turner 2, 3 , J S Tregoning 1, 2
Affiliation
|
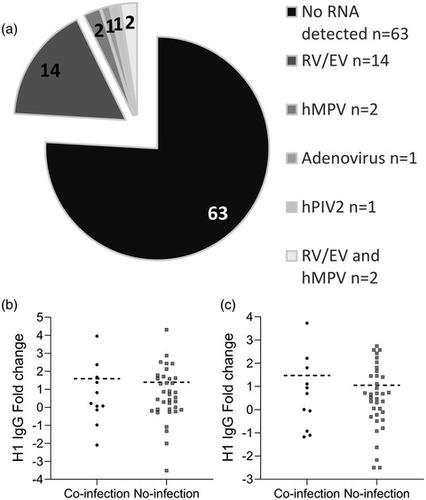
The United Kingdom has a national immunization programme which includes annual influenza vaccination in school‐aged children, using live attenuated influenza vaccine (LAIV). LAIV is given annually, and it is unclear whether repeat administration can affect immunogenicity. Because LAIV is delivered intranasally, pre‐existing local antibody might be important. In this study, we analysed banked samples from a study performed during the 2017/18 influenza season to investigate the role of pre‐existing influenza‐specific nasal immunoglobulin (Ig)A in children aged 6–14 years. Nasopharyngeal swabs were collected prior to LAIV immunization to measure pre‐existing IgA levels and test for concurrent upper respiratory tract viral infections (URTI). Oral fluid samples were taken at baseline and 21–28 days after LAIV to measure IgG as a surrogate of immunogenicity. Antibody levels at baseline were compared with a pre‐existing data set of LAIV shedding from the same individuals, measured by reverse transcription–polymerase chain reaction. There was detectable nasal IgA specific to all four strains in the vaccine at baseline. However, baseline nasal IgA did not correlate with the fold change in IgG response to the vaccine. Baseline nasal IgA also did not have an impact upon whether vaccine virus RNA was detectable after immunization. There was no difference in fold change of antibody between individuals with and without an URTI at the time of immunization. Overall, we observed no effect of pre‐existing influenza‐specific nasal antibody levels on immunogenicity, supporting annual immunization with LAIV in children.
中文翻译:

已有流感特异性鼻 IgA 或鼻病毒感染不会影响儿童流感减毒活疫苗的免疫原性
英国有一项国家免疫计划,其中包括每年使用减毒流感疫苗(LAIV)对学龄儿童进行流感疫苗接种。 LAIV 每年注射一次,目前尚不清楚重复注射是否会影响免疫原性。由于 LAIV 是鼻内给药,因此预先存在的局部抗体可能很重要。在这项研究中,我们分析了 2017/18 流感季节期间进行的一项研究的库存样本,以调查 6-14 岁儿童中预先存在的流感特异性鼻免疫球蛋白 (Ig)A 的作用。在 LAIV 免疫之前收集鼻咽拭子,以测量预先存在的 IgA 水平并测试并发的上呼吸道病毒感染 (URTI)。在基线和 LAIV 后 21-28 天采集口腔液样本,以测量 IgG 作为免疫原性的替代指标。通过逆转录聚合酶链反应测量基线抗体水平与同一个体先前存在的 LAIV 脱落数据集进行比较。基线时可检测到疫苗中所有四种毒株特异性的鼻 IgA。然而,基线鼻 IgA 与疫苗 IgG 反应的倍数变化无关。基线鼻 IgA 对免疫后是否可检测到疫苗病毒 RNA 也没有影响。免疫接种时有和没有 URTI 的个体之间抗体倍数变化没有差异。总体而言,我们观察到现有流感特异性鼻抗体水平对免疫原性没有影响,支持儿童每年接种 LAIV 疫苗。
更新日期:2020-12-13
中文翻译:

已有流感特异性鼻 IgA 或鼻病毒感染不会影响儿童流感减毒活疫苗的免疫原性
英国有一项国家免疫计划,其中包括每年使用减毒流感疫苗(LAIV)对学龄儿童进行流感疫苗接种。 LAIV 每年注射一次,目前尚不清楚重复注射是否会影响免疫原性。由于 LAIV 是鼻内给药,因此预先存在的局部抗体可能很重要。在这项研究中,我们分析了 2017/18 流感季节期间进行的一项研究的库存样本,以调查 6-14 岁儿童中预先存在的流感特异性鼻免疫球蛋白 (Ig)A 的作用。在 LAIV 免疫之前收集鼻咽拭子,以测量预先存在的 IgA 水平并测试并发的上呼吸道病毒感染 (URTI)。在基线和 LAIV 后 21-28 天采集口腔液样本,以测量 IgG 作为免疫原性的替代指标。通过逆转录聚合酶链反应测量基线抗体水平与同一个体先前存在的 LAIV 脱落数据集进行比较。基线时可检测到疫苗中所有四种毒株特异性的鼻 IgA。然而,基线鼻 IgA 与疫苗 IgG 反应的倍数变化无关。基线鼻 IgA 对免疫后是否可检测到疫苗病毒 RNA 也没有影响。免疫接种时有和没有 URTI 的个体之间抗体倍数变化没有差异。总体而言,我们观察到现有流感特异性鼻抗体水平对免疫原性没有影响,支持儿童每年接种 LAIV 疫苗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号