International Journal for Parasitology: Drugs and Drug Resistance ( IF 4.1 ) Pub Date : 2020-12-13 , DOI: 10.1016/j.ijpddr.2020.11.006 Aditi Arya 1 , Loick P Kojom Foko 1 , Shewta Chaudhry 1 , Amit Sharma 1 , Vineeta Singh 1
|
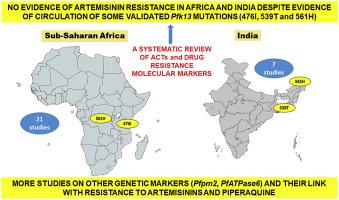
Artemisinin-based combination therapies (ACT) are currently used as a first-line malaria therapy in endemic countries worldwide. This systematic review aims at presenting the current scenario of drug resistance molecular markers, either selected or involved in treatment failures (TF) during in vivo ACT efficacy studies from sub-Saharan Africa (sSA) and India. Eight electronic databases were comprehensively used to search relevant articles and finally a total of 28 studies were included in the review, 21 from sSA and seven from India. On analysis, Artemether + lumefantrine (AL) and artesunate + sulfadoxine-pyrimethamine (AS + SP) are the main ACT in African and Indian regions with a 28-day efficacy range of 54.3–100% for AL and 63–100% for AS + SP respectively. It was observed that mutations in the Pfcrt (76T), Pfdhfr (51I, 59R, 108N), Pfdhps (437G) and Pfmdr1 (86Y, 184F, 1246Y) genes were involved in TF, which varied with respect to ACTs. Based on studies that have genotyped the Pfk13 gene, the reported TF cases, were mainly linked with mutations in genes associated with resistance to ACT partner drugs; indicating that the protection of the partner drug efficacy is crucial for maintaining the efficacy of ACT. This review reveals that ACT are largely efficacious in India and sSA despite the fact that some clinical efficacy and epidemiological studies have reported some validated mutations (i.e., 476I, 539T and 561H) in circulation in these two regions. Also, the role of PfATPase6 in ART resistance is controversial still, while P. falciparum plasmepsin 2 (Pfpm2) in piperaquine (PPQ) resistance and dihydroartemisinin (DHA) + PPQ failures is well documented in Southeast Asian countries but studied less in sSA. Hence, there is a need for continuous molecular surveillance of Pfk13 mutations for emergence of artemisinin (ART) resistance in these countries.
中文翻译:

基于青蒿素的联合疗法(ACT)和耐药分子标记:对印度和撒哈拉以南非洲两个疟疾流行地区的临床研究的系统评价
基于青蒿素的联合疗法(ACT)目前在全世界流行国家用作一线疟疾疗法。本系统综述旨在介绍撒哈拉以南非洲 (sSA) 和印度体内ACT 疗效研究期间选择或参与治疗失败 (TF) 的耐药分子标记的当前情况。综合利用8个电子数据库检索相关文章,最终共纳入28项研究,其中21项来自sSA,7项来自印度。据分析,蒿甲醚+苯芴醇(AL)和青蒿琥酯+磺胺多辛-乙胺嘧啶(AS+SP)是非洲和印度地区的主要ACT,28天疗效范围为AL为54.3-100%,AS为63-100%分别为+SP。据观察,TF涉及Pfcrt (76T)、Pfdhfr (51I、59R、108N)、Pfdhps (437G) 和Pfmdr1 (86Y、184F、1246Y) 基因的突变,其随 ACT 的变化而变化。根据对Pfk13基因进行基因分型的研究,报告的 TF 病例主要与 ACT 伙伴药物耐药相关基因的突变有关;表明保护伙伴药效对于维持ACT疗效至关重要。本次综述表明,尽管一些临床疗效和流行病学研究报告了这两个地区存在一些经过验证的突变(即 476I、539T 和 561H),但 ACT 在印度和南澳州基本上是有效的。此外, PfATPase6在 ART 耐药中的作用仍然存在争议,而恶性疟原虫血浆蛋白酶 2 (Pfpm2)在哌喹 (PPQ) 耐药和双氢青蒿素 (DHA) + PPQ 失败中的作用在东南亚国家已有详细记录,但在 sSA 中研究较少。因此,需要对Pfk13突变进行持续的分子监测,以了解这些国家是否出现青蒿素 (ART) 耐药性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号