Redox Biology ( IF 10.7 ) Pub Date : 2020-12-13 , DOI: 10.1016/j.redox.2020.101832 Arnab Ghosh 1 , Cynthia J Koziol-White 2 , William F Jester 2 , Serpil C Erzurum 1 , Kewal Asosingh 1 , Reynold A Panettieri 2 , Dennis J Stuehr 1
|
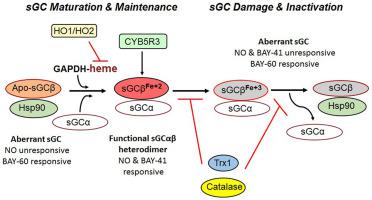
A subset of asthmatics develop a severe form of the disease whose etiology involves airway inflammation along with inherent drivers that remain ill-defined. To address this, we studied human airway smooth muscle cells (HASMC), whose relaxation drives airway bronchodilation and whose dysfunction contributes to airway obstruction and hypersensitivity in severe asthma. Because HASMC relaxation can be driven by the NO-soluble guanylyl cyclase (sGC)-cGMP signaling pathway, we questioned if HASMC from severe asthma donors might possess inherent defects in their sGC or in redox enzymes that support sGC function. We analyzed HASMC primary lines derived from 17 severe asthma and 16 normal donors and corresponding lung tissue samples regarding sGC activation by NO or by pharmacologic agonists, and also determined expression levels of sGC α1 and β1 subunits, supporting redox enzymes, and related proteins. We found a majority of the severe asthma donor HASMC (12/17) and lung samples primarily expressed a dysfunctional sGC that was NO-unresponsive and had low heterodimer content and high Hsp90 association. This sGC phenotype correlated with lower expression levels of the supporting redox enzymes cytochrome b5 reductase, catalase, and thioredoxin-1, and higher expression of heme oxygenases 1 and 2. Together, our work reveals that severe asthmatics are predisposed toward defective NO-sGC-cGMP signaling in their airway smooth muscle due to an inherent sGC dysfunction, which in turn is associated with inherent changes in the cell redox enzymes that impact sGC maturation and function.
中文翻译:

可溶性鸟苷酸环化酶的固有功能障碍存在于严重哮喘患者的气道中,并且与异常的氧化还原酶表达和受损的 NO-cGMP 信号传导有关
一部分哮喘患者发展为一种严重的疾病形式,其病因涉及气道炎症以及仍不明确的内在驱动因素。为了解决这个问题,我们研究了人气道平滑肌细胞 (HASMC),其松弛会导致气道支气管扩张,其功能障碍会导致严重哮喘患者的气道阻塞和超敏反应。因为 HASMC 松弛可由 NO 可溶性鸟苷酸环化酶 (sGC)-cGMP 信号通路驱动,我们质疑来自严重哮喘供体的 HASMC 是否可能在其 sGC 或支持 sGC 功能的氧化还原酶中存在固有缺陷。我们分析了来自 17 名严重哮喘患者和 16 名正常供体的 HASMC 原代细胞系和相应的肺组织样本,了解 NO 或药物激动剂对 sGC 的激活情况,并确定了 sGC α1 和 β1 亚基的表达水平,支持氧化还原酶和相关蛋白质。我们发现大多数重度哮喘供体 HASMC (12/17) 和肺样本主要表达功能失调的 sGC,它对 NO 无反应,异二聚体含量低,Hsp90 结合率高。这种 sGC 表型与支持性氧化还原酶细胞色素的较低表达水平相关b 5 还原酶、过氧化氢酶和硫氧还蛋白-1,以及血红素加氧酶 1 和 2 的更高表达。我们的研究表明,由于固有的 sGC 功能障碍,重度哮喘患者的气道平滑肌中的 NO-sGC-cGMP 信号传导有缺陷的倾向,这反过来又与影响 sGC 成熟和功能的细胞氧化还原酶的固有变化有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号