当前位置:
X-MOL 学术
›
Polym. Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polyetheretherketone implant surface functionalization technologies and the need for a transparent quality evaluation system
Polymer International ( IF 2.9 ) Pub Date : 2020-12-10 , DOI: 10.1002/pi.6162 Dietmar Schaffarczyk 1, 2 , Jennifer Knaus 2 , Gunther Peeters 2 , Dieter Scholl 1 , Andreas Schwitalla 3 , Christoph Koslowski 4 , Helmut Cölfen 5
Polymer International ( IF 2.9 ) Pub Date : 2020-12-10 , DOI: 10.1002/pi.6162 Dietmar Schaffarczyk 1, 2 , Jennifer Knaus 2 , Gunther Peeters 2 , Dieter Scholl 1 , Andreas Schwitalla 3 , Christoph Koslowski 4 , Helmut Cölfen 5
Affiliation
|
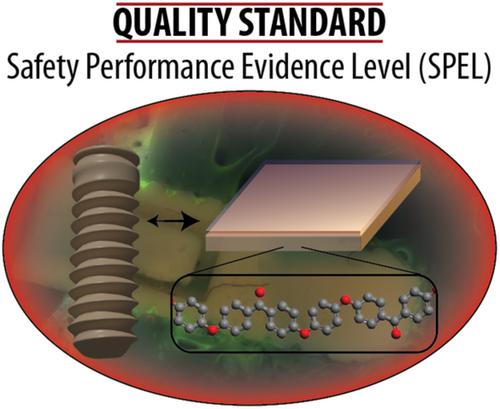
For bone implants, osseointegration resulting in a good and fast bone–implant contact is of primary importance to secure a proper implant function and to avoid implant loosening or inflammation resulting in necessary revision surgeries causing pain to the patients and immense costs. In particular, polyetheretherketone (PEEK) is a promising implant material due to the close mechanical properties to bone, but it is entirely bio-inert, hindering osseointegration and making surface functionalization necessary. Many different surface functionalization technologies have been reported of both physical and chemical nature. The same is true for the other prominent implant materials titanium and ceramics. Although they already have inherently better osseointegration than PEEK, they are much harder and stiffer than bone and brittle in the case of ceramics. Surface functionalization, which can be subdivided into surface coating and material modification, needs to be judged from a quality and safety viewpoint. However, a literature research resulted in the realization that no quality standard yet exists for implant surface functionalizations. This makes it difficult to near impossible to compare the safety and performance of different surface-functionalized bone implants, clearly showing the need to establish a transparent quality evaluation system for bone implants. This perspective article gives the state of the art and then develops a quality evaluation system based on six main categories as important benchmarks for the quality of surface-functionalized bone implant materials. A simple catalog of questions can be answered, and from the resulting scores the Safety and Performance Evidence Level (SPEL) representing the safety and quality of a given implant can be calculated as a percentage. This simple SPEL system allows an easy and transparent judgment and comparison of bone implants, ensuring the easy identification of safe and well-performing high-quality bone implants in the future. © 2020 The Authors. Polymer International published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
中文翻译:

聚醚醚酮植入物表面功能化技术和对透明质量评估系统的需求
对于骨植入物,骨结合导致良好和快速的骨 - 植入物接触对于确保适当的植入物功能并避免植入物松动或炎症导致必要的修复手术导致患者疼痛和巨额成本至关重要。特别是,聚醚醚酮 (PEEK) 是一种很有前途的植入材料,因为它与骨骼的机械性能非常接近,但它完全是生物惰性的,阻碍了骨整合并使表面功能化成为必要。许多不同的表面功能化技术已经报道了物理和化学性质。其他突出的植入材料钛和陶瓷也是如此。尽管它们在本质上已经比 PEEK 具有更好的骨整合,但它们比骨骼更坚硬、更坚硬,而且在陶瓷的情况下更脆。表面功能化可以细分为表面涂层和材料改性,需要从质量和安全的角度来判断。然而,一项文献研究表明,目前还没有种植体表面功能化的质量标准。这使得比较不同表面功能化骨植入物的安全性和性能变得几乎不可能,这清楚地表明需要建立一个透明的骨植入物质量评估体系。这篇透视文章给出了最新技术,然后开发了一个基于六个主要类别的质量评估系统,作为表面功能化骨植入材料质量的重要基准。可以回答一个简单的问题目录,并且根据得到的分数,代表给定植入物的安全性和质量的安全性和性能证据水平 (SPEL) 可以计算为百分比。这种简单的 SPEL 系统可以轻松透明地判断和比较骨植入物,确保将来轻松识别安全且性能良好的高质量骨植入物。© 2020 作者。由 John Wiley & Sons Ltd 代表化学工业协会出版的Polymer International。
更新日期:2020-12-10
中文翻译:

聚醚醚酮植入物表面功能化技术和对透明质量评估系统的需求
对于骨植入物,骨结合导致良好和快速的骨 - 植入物接触对于确保适当的植入物功能并避免植入物松动或炎症导致必要的修复手术导致患者疼痛和巨额成本至关重要。特别是,聚醚醚酮 (PEEK) 是一种很有前途的植入材料,因为它与骨骼的机械性能非常接近,但它完全是生物惰性的,阻碍了骨整合并使表面功能化成为必要。许多不同的表面功能化技术已经报道了物理和化学性质。其他突出的植入材料钛和陶瓷也是如此。尽管它们在本质上已经比 PEEK 具有更好的骨整合,但它们比骨骼更坚硬、更坚硬,而且在陶瓷的情况下更脆。表面功能化可以细分为表面涂层和材料改性,需要从质量和安全的角度来判断。然而,一项文献研究表明,目前还没有种植体表面功能化的质量标准。这使得比较不同表面功能化骨植入物的安全性和性能变得几乎不可能,这清楚地表明需要建立一个透明的骨植入物质量评估体系。这篇透视文章给出了最新技术,然后开发了一个基于六个主要类别的质量评估系统,作为表面功能化骨植入材料质量的重要基准。可以回答一个简单的问题目录,并且根据得到的分数,代表给定植入物的安全性和质量的安全性和性能证据水平 (SPEL) 可以计算为百分比。这种简单的 SPEL 系统可以轻松透明地判断和比较骨植入物,确保将来轻松识别安全且性能良好的高质量骨植入物。© 2020 作者。由 John Wiley & Sons Ltd 代表化学工业协会出版的Polymer International。










































 京公网安备 11010802027423号
京公网安备 11010802027423号