当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Directed Electron Transfer in Flavin Peptides with Oligoproline‐Type Helical Conformation as Models for Flavin‐Functional Proteins
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-12-11 , DOI: 10.1002/open.202000199 Samantha Wörner 1 , Julia Leier 2 , Nadine C Michenfelder 2 , Andreas-Neil Unterreiner 2 , Hans-Achim Wagenknecht 1
ChemistryOpen ( IF 2.3 ) Pub Date : 2020-12-11 , DOI: 10.1002/open.202000199 Samantha Wörner 1 , Julia Leier 2 , Nadine C Michenfelder 2 , Andreas-Neil Unterreiner 2 , Hans-Achim Wagenknecht 1
Affiliation
|
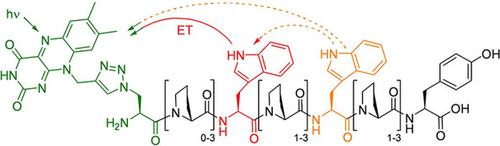
To mimic the charge separation in functional proteins we studied flavin‐modified peptides as models. They were synthesized as oligoprolines that typically form a polyproline type‐II helix, because this secondary structure supports the electron transfer properties. We placed the flavin as photoexcitable chromophore and electron acceptor at the N‐terminus. Tryptophans were placed as electron donors to direct the electron transfer over 0–3 intervening prolines. Spectroscopic studies revealed competitive photophysical pathways. The reference peptide without tryptophan shows dominant non‐specific ET dynamics, leading to an ion pair formation, whereas peptides with tryptophans have weak non‐specific ET and intensified directed electron transfer. By different excitation wavelengths, we can conclude that the corresponding ion pair state of flavin within the peptide environment has to be energetically located between the S1 and S4 states, whereas the directed electron transfer to tryptophan occurs directly from the S1 state. These photochemical results have fundamental significance for proteins with flavin as redoxactive cofactor.
中文翻译:

具有寡脯氨酸型螺旋构象的黄素肽中的定向电子转移作为黄素功能蛋白的模型
为了模拟功能蛋白质中的电荷分离,我们研究了黄素修饰的肽作为模型。它们被合成为通常形成聚脯氨酸 II 型螺旋的低聚脯氨酸,因为这种二级结构支持电子转移特性。我们将黄素作为可光激发的发色团和电子受体放置在 N 端。色氨酸被放置为电子供体,以指导电子转移超过 0-3 间脯氨酸。光谱研究揭示了竞争性光物理途径。不含色氨酸的参考肽显示出主要的非特异性 ET 动力学,导致离子对形成,而含有色氨酸的肽具有弱的非特异性 ET 和强化的定向电子转移。通过不同的激发波长,1和 S 4状态,而向色氨酸的定向电子转移直接从 S 1状态发生。这些光化学结果对于以黄素为氧化还原活性辅因子的蛋白质具有重要意义。
更新日期:2020-12-12
中文翻译:

具有寡脯氨酸型螺旋构象的黄素肽中的定向电子转移作为黄素功能蛋白的模型
为了模拟功能蛋白质中的电荷分离,我们研究了黄素修饰的肽作为模型。它们被合成为通常形成聚脯氨酸 II 型螺旋的低聚脯氨酸,因为这种二级结构支持电子转移特性。我们将黄素作为可光激发的发色团和电子受体放置在 N 端。色氨酸被放置为电子供体,以指导电子转移超过 0-3 间脯氨酸。光谱研究揭示了竞争性光物理途径。不含色氨酸的参考肽显示出主要的非特异性 ET 动力学,导致离子对形成,而含有色氨酸的肽具有弱的非特异性 ET 和强化的定向电子转移。通过不同的激发波长,1和 S 4状态,而向色氨酸的定向电子转移直接从 S 1状态发生。这些光化学结果对于以黄素为氧化还原活性辅因子的蛋白质具有重要意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号