Tetrahedron Letters ( IF 1.5 ) Pub Date : 2020-12-11 , DOI: 10.1016/j.tetlet.2020.152707 Ruonan Ma , Xueyuan Chen , Zhiyin Xiao , Mookan Natarajan , Chunxin Lu , Xiujuan Jiang , Wei Zhong , Xiaoming Liu
|
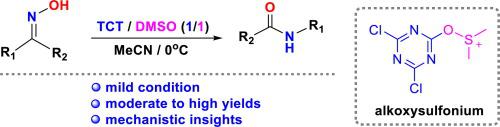
Synthesis of amides via Beckmann rearrangement of ketoximes promoted by cyanuric chloride (TCT)/DMSO under mild conditions has been reported. Conditions of the Beckmann rearrangement, e.g., solvents, the ratios of TCT/DMSO, and the temperature, were investigated using diphenylmethanone oxime as a substrate. The optimized conditions were adopted to afford fourteen amides with yields ranging from 20% to 99%. A plausible mechanism involving an active dimethyl alkoxysulfonium intermediate was proposed according to the mass spectrometry analysis. To our best knowledge, this is the first case of study on Beckmann rearrangement of ketoximes promoted by TCT/DMSO under a mild condition to afford amides efficiently.
中文翻译:

氰尿酰氯和二甲基亚砜在温和条件下促进酮肟的贝克曼重排
酰胺的合成通过在温和条件下DMSO已经报道/由氰尿酰氯(TCT)促进酮肟的贝克曼重排。使用二苯甲酮肟作为底物,研究贝克曼重排的条件,例如溶剂,TCT / DMSO的比例和温度。采用优化的条件得到十四种酰胺,收率范围为20%至99%。根据质谱分析,提出了一种涉及活性二甲基烷氧基s中间体的合理机理。据我们所知,这是第一个研究TCT / DMSO在温和条件下促进酮肟的贝克曼重排以有效提供酰胺的案例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号