Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-12-11 , DOI: 10.1016/j.bbamem.2020.183532 Yoshiaki Yano 1 , Yuta Watanabe 1 , Katsumi Matsuzaki 1
|
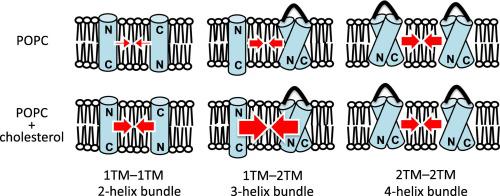
The tertiary structures and conformational dynamics of transmembrane (TM) helical proteins are maintained by the interhelical interaction network in membranes, although it is complicated to analyze the underlying driving forces because the amino acid sequences can involve multiple and various types of interactions. To obtain insights into basal and common effects of the number of membrane-spanning segments and membrane cholesterol, we measured stabilities of helix bundles composed of simple TM helices (AALALAA)3 (1TM) and (AALALAA)3-G5-(AALALAA)3 (2TM). Association–dissociation dynamics for 1TM–1TM, 1TM–2TM, and 2TM–2TM pairs were monitored to compare stabilities of 2-, 3-, and 4-helical bundles, respectively, with single-pair fluorescence resonance energy transfer (sp-FRET) in liposome membranes. Both thermodynamic and kinetic stabilities of the helix bundles increased with a greater number of membrane-spanning segments in POPC. The presence of 30 mol% cholesterol strongly enhanced the formation of 1TM–1TM and 1TM–2TM bundles (~ − 9 kJ mol−1), whereas it only weakly stabilized the 2TM–2TM bundle (~ − 3 kJ mol−1). Fourier transform infrared-polarized attenuated total reflection (ATR-FTIR) spectroscopy revealed an ~30° tilt of the helix axis relative to bilayer normal for the 1TM–2TM pair in the presence of cholesterol, suggesting the formation of a tilted helix bundle to release high lateral pressure at the center of cholesterol-containing membranes. These results demonstrate that the number of membrane-spanning segments affects the stability and structure of the helix bundle, and their cholesterol-dependences. Such information is useful to understand the basics of folding and assembly of multispanning TM proteins.
中文翻译:

单对FRET分析显示跨膜螺旋束的热力学和动力学稳定性:跨膜片段数和胆固醇的影响
跨膜(TM)螺旋蛋白的三级结构和构象动力学由膜中的螺旋间相互作用网络维持,尽管分析潜在的驱动力很复杂,因为氨基酸序列可能涉及多种不同类型的相互作用。为了深入了解跨膜节段数和膜胆固醇的基础和常见作用,我们测量了由简单TM螺旋(AALALAA)3(1TM)和(AALALAA)3 -G 5-(AALALAA)组成的螺旋束的稳定性3(2TM)。监测了1TM-1TM,1TM-2TM和2TM-2TM对的缔合-解离动力学,分别比较了2、3和4螺旋束的稳定性,以及单对荧光共振能量转移(sp-FRET )在脂质体膜中。螺旋束的热力学和动力学稳定性都随着POPC中跨膜段数量的增加而增加。30 mol%胆固醇的存在强烈增强了1TM–1TM和1TM–2TM束的形成(〜-9 kJ mol -1),而它只能使2TM–2TM束(〜− 3 kJ mol -1)稳定下来。)。傅立叶变换红外偏振衰减全反射(ATR-FTIR)光谱显示,在胆固醇存在的情况下,相对于1TM–2TM对的双层法线,螺旋轴相对于双层法线有约30°的倾斜,表明释放了倾斜的螺旋束含胆固醇膜中心的侧向压力高。这些结果表明跨膜区段的数目影响螺旋束的稳定性和结构,以及它们对胆固醇的依赖性。这样的信息对于理解多跨TM蛋白的折叠和组装基础非常有用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号