当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Orientation of the α-CD component of [2]rotaxanes affects their specific molecular recognition behaviour
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-11-26 , DOI: 10.1039/d0qo01337d Takuya Iwamoto 1, 2, 3, 4, 5 , Shinobu Miyagawa 1, 2, 3, 4, 5 , Masaya Naito 1, 2, 3, 4, 5 , Yuji Tokunaga 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-11-26 , DOI: 10.1039/d0qo01337d Takuya Iwamoto 1, 2, 3, 4, 5 , Shinobu Miyagawa 1, 2, 3, 4, 5 , Masaya Naito 1, 2, 3, 4, 5 , Yuji Tokunaga 1, 2, 3, 4, 5
Affiliation
|
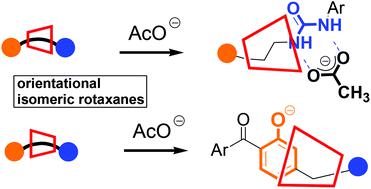
In this study we synthesized a pair of orientationally isomeric [2]rotaxanes (1 and 2) featuring an α-cyclodextrin (α-CD) as the macrocyclic component and phenylurea and 2-hydroxybenzophenone moieties as stoppers of the axle-like component. The presence of the α-CD component enhanced the anion recognition ability of the urea unit in isomer 1, with the OH groups on the wide rim of α-CD undergoing hydrogen bonding with the anions. The association constant for the interaction between 1 and the acetate (AcO−) anion was more than four times that of the corresponding non-interlocked dumbbell-shaped molecule and the AcO− anion. The other isomer 2, in which the wide rim of the α-CD component faced the 2-hydroxybenzophenone unit, was preferentially deprotonated by the AcO− anion to give the phenoxide anion, which was stabilized through noncovalent interactions with the OH groups on the wide rim of the α-CD unit in an organic solvent; in contrast, the AcO− anion did not deprotonate the corresponding non-interlocked dumbbell-shaped molecule.
中文翻译:

[2]轮烷的α-CD成分的取向影响其特定的分子识别行为
在这项研究中,我们合成了一对定向异构的[2]轮烷(1和2),其特征在于α-环糊精(α-CD)作为大环成分,苯基脲和2-羟基二苯甲酮部分作为轴状成分的塞子。α-CD组分的存在增强了异构体1中尿素单元的阴离子识别能力,α-CD宽边上的OH基团与阴离子发生氢键结合。对之间的相互作用的缔合常数1和醋酸(ACO - )阴离子是相应的非互锁哑铃状分子和ACO的四倍以上-的阴离子。其他异构体2,其中,所述α-CD分量的宽边沿面对的2-羟基二苯甲酮单元,由ACO被优先去质子化-阴离子,得到酚盐阴离子,其是通过与OH基团的非共价相互作用的α的宽边沿稳定-CD单元在有机溶剂中;与此相反,ACO -阴离子没有去质子化相应的非互锁哑铃形分子。
更新日期:2020-12-17
中文翻译:

[2]轮烷的α-CD成分的取向影响其特定的分子识别行为
在这项研究中,我们合成了一对定向异构的[2]轮烷(1和2),其特征在于α-环糊精(α-CD)作为大环成分,苯基脲和2-羟基二苯甲酮部分作为轴状成分的塞子。α-CD组分的存在增强了异构体1中尿素单元的阴离子识别能力,α-CD宽边上的OH基团与阴离子发生氢键结合。对之间的相互作用的缔合常数1和醋酸(ACO - )阴离子是相应的非互锁哑铃状分子和ACO的四倍以上-的阴离子。其他异构体2,其中,所述α-CD分量的宽边沿面对的2-羟基二苯甲酮单元,由ACO被优先去质子化-阴离子,得到酚盐阴离子,其是通过与OH基团的非共价相互作用的α的宽边沿稳定-CD单元在有机溶剂中;与此相反,ACO -阴离子没有去质子化相应的非互锁哑铃形分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号