当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Comparative Craniofacial Bone Regeneration Capacities of Mesenchymal Stem Cells Derived from Human Neural Crest Stem Cells and Bone Marrow
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-12-09 , DOI: 10.1021/acsbiomaterials.0c00878 Akshaya Srinivasan 1, 2, 3 , Nelson Teo 4 , Kei Jun Poon 4 , Priya Tiwari 5 , Akhilandeshwari Ravichandran 6, 7 , Feng Wen 6 , Swee Hin Teoh 6 , Thiam Chye Lim 4, 5 , Yi-Chin Toh 1, 3, 7, 8, 9
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2020-12-09 , DOI: 10.1021/acsbiomaterials.0c00878 Akshaya Srinivasan 1, 2, 3 , Nelson Teo 4 , Kei Jun Poon 4 , Priya Tiwari 5 , Akhilandeshwari Ravichandran 6, 7 , Feng Wen 6 , Swee Hin Teoh 6 , Thiam Chye Lim 4, 5 , Yi-Chin Toh 1, 3, 7, 8, 9
Affiliation
|
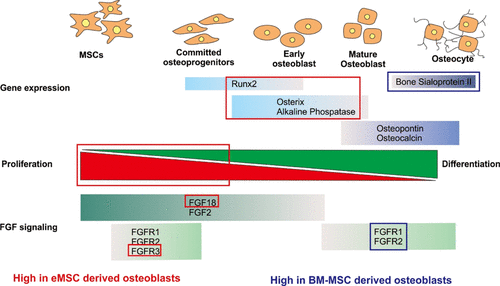
Most craniofacial bones are derived from the ectodermal germ layer via neural crest stem cells, which are distinct from mesoderm-derived long bones. However, current craniofacial bone tissue engineering approaches do not account for this difference and utilize mesoderm-derived bone marrow mesenchymal stem cells (BM-MSCs) as a paradigm cell source. The effect of the embryonic origin (ontogeny) of an MSC population on its osteogenic differentiation potential and regenerative ability still remains unresolved. To clarify the effects of MSC ontogeny on bone regenerative ability, we directly compared the craniofacial bone regenerative abilities of an ecto-mesenchymal stem cell (eMSC) population, which is derived from human embryonic stem cells via a neural crest intermediate, with mesodermal adult BM-MSCs. eMSCs showed comparable osteogenic and chondrogenic ability to BM-MSCs in 2-D in vitro culture, but lower adipogenic ability. They exhibited greater proliferation than BM-MSCs and comparable construct mineralization in a well-established 3-D polycaprolactone-tricalcium phosphate (PCL-TCP) scaffold system in vitro. eMSC-derived 3D osteogenic constructs were maintained for longer in a proliferative osteoblast state and exhibited differential levels of genes related to fibroblast growth factor (FGF) signaling compared to BM-MSCs. Although both eMSC and BM-MSC-seeded scaffold constructs could promote bone regeneration in a rat calvarial defect model, eMSC-derived osseous constructs had significantly higher cellularity due to increased number of proliferative (Ki67+) cells than those seeded with BM-MSCs, and exhibited enhanced new bone formation in the defect area as compared to untreated controls. Overall, our study demonstrates the potential of human eMSCs for future clinical use in craniofacial regeneration applications and indicates the importance of considering MSC origin when selecting an MSC source for regenerative applications.
中文翻译:

来自人神经Ne干细胞和骨髓的间充质干细胞的颅面骨再生能力的比较
大多数颅面骨是通过神经c干细胞从外胚层胚层中获得的,而神经from干细胞不同于中胚层衍生的长骨。但是,当前的颅面骨组织工程方法不能解决这一差异,而是利用中胚层衍生的骨髓间充质干细胞(BM-MSC)作为范例细胞来源。MSC种群的胚胎起源(个体发育)对其成骨分化潜能和再生能力的影响仍未解决。为了阐明MSC个体发育对骨再生能力的影响,我们直接比较了人间充质干细胞(eMSC)人群的颅面骨再生能力,该细胞是通过人类胚胎干细胞通过神经rest中间体,具有成年中胚层BM-MSC。eMSCs在二维体外培养中显示出与BM-MSCs相当的成骨和软骨形成能力,但成脂能力较低。它们在成熟的3-D聚己内酯-磷酸三钙(PCL-TCP)支架系统中表现出比BM-MSC更大的增殖能力和可比的构建体矿化作用。与BM-MSC相比,eMSC衍生的3D成骨构建体在增生的成骨细胞状态下可维持更长的时间,并且显示出与成纤维细胞生长因子(FGF)信号相关的基因水平不同。尽管eMSC和BM-MSC种植的支架构建体均可在大鼠颅盖骨缺损模型中促进骨再生,但eMSC衍生的骨构建体由于增殖(Ki67 +)细胞比接种BM-MSC的细胞多,并且与未处理的对照组相比,在缺损区域表现出增强的新骨形成。总体而言,我们的研究证明了人类eMSC在颅面再生应用中未来临床应用的潜力,并指出了在选择用于再生应用的MSC来源时考虑MSC来源的重要性。
更新日期:2021-01-11
中文翻译:

来自人神经Ne干细胞和骨髓的间充质干细胞的颅面骨再生能力的比较
大多数颅面骨是通过神经c干细胞从外胚层胚层中获得的,而神经from干细胞不同于中胚层衍生的长骨。但是,当前的颅面骨组织工程方法不能解决这一差异,而是利用中胚层衍生的骨髓间充质干细胞(BM-MSC)作为范例细胞来源。MSC种群的胚胎起源(个体发育)对其成骨分化潜能和再生能力的影响仍未解决。为了阐明MSC个体发育对骨再生能力的影响,我们直接比较了人间充质干细胞(eMSC)人群的颅面骨再生能力,该细胞是通过人类胚胎干细胞通过神经rest中间体,具有成年中胚层BM-MSC。eMSCs在二维体外培养中显示出与BM-MSCs相当的成骨和软骨形成能力,但成脂能力较低。它们在成熟的3-D聚己内酯-磷酸三钙(PCL-TCP)支架系统中表现出比BM-MSC更大的增殖能力和可比的构建体矿化作用。与BM-MSC相比,eMSC衍生的3D成骨构建体在增生的成骨细胞状态下可维持更长的时间,并且显示出与成纤维细胞生长因子(FGF)信号相关的基因水平不同。尽管eMSC和BM-MSC种植的支架构建体均可在大鼠颅盖骨缺损模型中促进骨再生,但eMSC衍生的骨构建体由于增殖(Ki67 +)细胞比接种BM-MSC的细胞多,并且与未处理的对照组相比,在缺损区域表现出增强的新骨形成。总体而言,我们的研究证明了人类eMSC在颅面再生应用中未来临床应用的潜力,并指出了在选择用于再生应用的MSC来源时考虑MSC来源的重要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号