当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Halophilic to mesophilic adaptation of ubiquitin‐like proteins
FEBS Letters ( IF 3.0 ) Pub Date : 2020-12-19 , DOI: 10.1002/1873-3468.14023 Quan Li 1, 2 , Mengqing Li 1, 2 , Cong Li 3 , Xinxin Li 1, 2 , Chenghui Lu 1, 2 , Xiaoming Tu 3 , Zhiyong Zhang 3 , Xuecheng Zhang 1, 2
FEBS Letters ( IF 3.0 ) Pub Date : 2020-12-19 , DOI: 10.1002/1873-3468.14023 Quan Li 1, 2 , Mengqing Li 1, 2 , Cong Li 3 , Xinxin Li 1, 2 , Chenghui Lu 1, 2 , Xiaoming Tu 3 , Zhiyong Zhang 3 , Xuecheng Zhang 1, 2
Affiliation
|
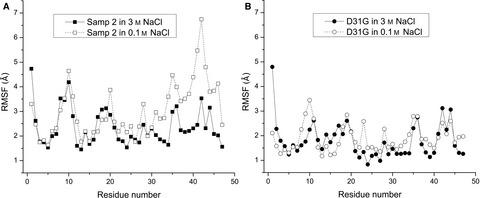
Elucidating how proteins adapt from halophilic to mesophilic environments will enable a better understanding of protein evolution and folding. In this study, by directed evolution and site-directed mutagenesis of the halophilic ubiquitin-like protein (ULP) Samp2, we find that substitution of the prebiotic amino acid Asp31 by Gly is uniquely effective in the mesophilic adaptation of ULP. Sequence analysis shows that substitution of Asp/Glu in halophilic ULPs by Gly in mesophilic ULPs has higher occurrence than other substitutions, supporting the unique role of the substitution in the mesophilic adaptation of ULP. Molecular dynamics simulations indicate that the mesophilic adaptation might result from the effect of the substitution on the conformational flexibility of ULP.
中文翻译:

泛素样蛋白的嗜盐到嗜温适应
阐明蛋白质如何从嗜盐环境适应到嗜温环境将有助于更好地了解蛋白质的进化和折叠。在这项研究中,通过嗜盐泛素样蛋白 (ULP) Samp2 的定向进化和定点诱变,我们发现 Gly 取代益生元氨基酸 Asp31 在 ULP 的嗜温适应中具有独特的效果。序列分析表明,嗜盐 ULP 中的 Asp/Glu 被嗜温 ULP 中的 Gly 取代的发生率高于其他取代,支持该取代在 ULP 嗜温适应中的独特作用。分子动力学模拟表明,中温适应可能是由于取代对 ULP 构象灵活性的影响。
更新日期:2020-12-19
中文翻译:

泛素样蛋白的嗜盐到嗜温适应
阐明蛋白质如何从嗜盐环境适应到嗜温环境将有助于更好地了解蛋白质的进化和折叠。在这项研究中,通过嗜盐泛素样蛋白 (ULP) Samp2 的定向进化和定点诱变,我们发现 Gly 取代益生元氨基酸 Asp31 在 ULP 的嗜温适应中具有独特的效果。序列分析表明,嗜盐 ULP 中的 Asp/Glu 被嗜温 ULP 中的 Gly 取代的发生率高于其他取代,支持该取代在 ULP 嗜温适应中的独特作用。分子动力学模拟表明,中温适应可能是由于取代对 ULP 构象灵活性的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号