当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Voltammetry and Single‐Molecule In Situ Scanning Tunnelling Microscopy of the Redox Metalloenzyme Human Sulfite Oxidase
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-12-10 , DOI: 10.1002/celc.202001258 Jiawei Yan 1, 2 , Emil Egede Frøkjær 1 , Christian Engelbrekt 1 , Silke Leimkühler 3 , Jens Ulstrup 1 , Ulla Wollenberger 3 , Xinxin Xiao 1 , Jingdong Zhang 1
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-12-10 , DOI: 10.1002/celc.202001258 Jiawei Yan 1, 2 , Emil Egede Frøkjær 1 , Christian Engelbrekt 1 , Silke Leimkühler 3 , Jens Ulstrup 1 , Ulla Wollenberger 3 , Xinxin Xiao 1 , Jingdong Zhang 1
Affiliation
|
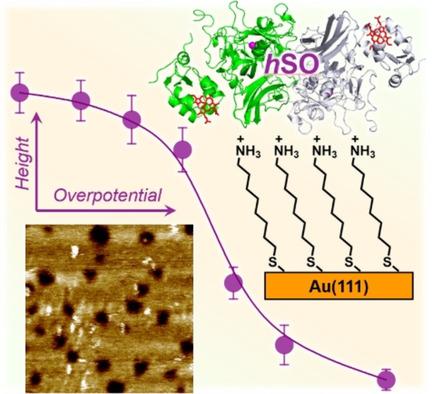
Human sulfite oxidase (hSO) is a homodimeric two‐domain enzyme central in the biological sulfur cycle. A pyranopterin molybdenum cofactor (Moco) is the catalytic site and a heme b5 group located in the N‐terminal domain. The two domains are connected by a flexible linker region. Electrons produced at the Moco in sulfite oxidation, are relayed via heme b5 to electron acceptors or an electrode surface. Inter‐domain conformational changes between an open and a closed enzyme conformation, allowing “gated” electron transfer has been suggested. We first recorded cyclic voltammetry (CV) of hSO on single‐crystal Au(111)‐electrode surfaces modified by self‐assembled monolayers (SAMs) both of a short rigid thiol, cysteamine and of a longer structurally flexible thiol, ω‐amino‐octanethiol (AOT). hSO on cysteamine SAMs displays a well‐defined pair of voltammetric peaks around −0.207 V vs. SCE in the absence of sulfite substrate, but no electrocatalysis. hSO on AOT SAMs displays well‐defined electrocatalysis, but only “fair” quality voltammetry in the absence of sulfite. We recorded next in situ scanning tunnelling spectroscopy (STS) of hSO on AOT modified Au(111)‐electrodes, disclosing, a 2–5 % surface coverage of strong molecular scale contrasts, assigned to single hSO molecules, notably with no contrast difference in the absence and presence of sulfite. In situ STS corroborated this observation with a sigmoidal tunnelling current/overpotential correlation.
中文翻译:

氧化还原金属酶人类亚硫酸盐氧化酶的伏安法和单分子原位扫描隧道显微镜
人亚硫酸盐氧化酶(h SO)是生物二硫循环中重要的同型二聚体双域酶。吡喃蝶呤钼辅助因子(Moco)是催化位点,且位于N末端域的血红素b 5基团。这两个域通过灵活的链接器区域连接。Moco在亚硫酸盐氧化反应中产生的电子通过血红素b 5传递到电子受体或电极表面。建议在开放和封闭的酶构象之间进行域间构象变化,从而实现“门控”电子转移。我们首先记录了h的循环伏安法(CV)单晶Au(111)电极表面上的SO受到短刚性硬硫醇,半胱胺和结构较长的柔性硫醇ω-氨基辛硫醇(AOT)的自组装单层(SAM)修饰。在没有亚硫酸盐底物但没有电催化的情况下,半胱胺SAM上的h SO在-0.207 V相对于SCE的位置上显示了一对明确定义的伏安峰。在AOT SAM上的h SO表现出明确的电催化作用,但是在没有亚硫酸盐的情况下只有“中等”质量伏安法。我们在AOT修饰的Au(111)-电极上记录了h SO的下一次原位扫描隧道光谱(STS),揭示了2–5%的强分子尺度对比的表面覆盖率,分配给单个hSO分子,特别是在不存在和存在亚硫酸盐的情况下,没有对比差异。原位STS以S形隧道电流/过电势相关性证实了这一观察结果。
更新日期:2021-01-04
中文翻译:

氧化还原金属酶人类亚硫酸盐氧化酶的伏安法和单分子原位扫描隧道显微镜
人亚硫酸盐氧化酶(h SO)是生物二硫循环中重要的同型二聚体双域酶。吡喃蝶呤钼辅助因子(Moco)是催化位点,且位于N末端域的血红素b 5基团。这两个域通过灵活的链接器区域连接。Moco在亚硫酸盐氧化反应中产生的电子通过血红素b 5传递到电子受体或电极表面。建议在开放和封闭的酶构象之间进行域间构象变化,从而实现“门控”电子转移。我们首先记录了h的循环伏安法(CV)单晶Au(111)电极表面上的SO受到短刚性硬硫醇,半胱胺和结构较长的柔性硫醇ω-氨基辛硫醇(AOT)的自组装单层(SAM)修饰。在没有亚硫酸盐底物但没有电催化的情况下,半胱胺SAM上的h SO在-0.207 V相对于SCE的位置上显示了一对明确定义的伏安峰。在AOT SAM上的h SO表现出明确的电催化作用,但是在没有亚硫酸盐的情况下只有“中等”质量伏安法。我们在AOT修饰的Au(111)-电极上记录了h SO的下一次原位扫描隧道光谱(STS),揭示了2–5%的强分子尺度对比的表面覆盖率,分配给单个hSO分子,特别是在不存在和存在亚硫酸盐的情况下,没有对比差异。原位STS以S形隧道电流/过电势相关性证实了这一观察结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号