当前位置:
X-MOL 学术
›
Mol. Syst. Des. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A detailed computational study on binding of kinase inhibitors into β-cyclodextrin: inclusion complex formation
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0me00140f Maryam Faraj Pour Mojdehi 1, 2, 3, 4, 5 , Mokhtar Ganjali Koli 1, 2, 3, 4, 5 , Mahsa Dolatkhah Ouch Bolagh 1, 2, 3, 4, 5 , Mina Ghane Gardeh 1, 2, 3, 4, 5 , Seyed Majid Hashemianzadeh 1, 2, 3, 4, 5
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2020-12-8 , DOI: 10.1039/d0me00140f Maryam Faraj Pour Mojdehi 1, 2, 3, 4, 5 , Mokhtar Ganjali Koli 1, 2, 3, 4, 5 , Mahsa Dolatkhah Ouch Bolagh 1, 2, 3, 4, 5 , Mina Ghane Gardeh 1, 2, 3, 4, 5 , Seyed Majid Hashemianzadeh 1, 2, 3, 4, 5
Affiliation
|
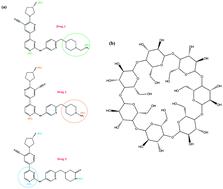
It is well known that the limited aqueous solubility of some drugs often reduces their bioavailability to targets. Inclusion complex formation of drugs with β-cyclodextrin is one of the best approaches for drug delivery improvement. Extensive microscopic molecular dynamics simulations were performed to study the interactions between β-cyclodextrin and a class of kinase inhibitor drugs. Solvation free energy calculations demonstrated that these drugs are insoluble in water and reinforced the need for a carrier to increase the solubility. The binding of these drugs into β-cyclodextrin was assessed by calculating the binding free energy, and it was found that all drugs tended to be bound thermodynamically. Examination of the dynamic properties showed that the drugs were loaded into β-cyclodextrin, so due to the loading of drugs into the β-cyclodextrin, the mean square displacement (MSD) of all the drugs drastically decreased. The loading of drugs was accompanied by pushing of water out of the center of the β-cyclodextrin cavity. The drugs showed different orientations and mechanisms for loading into β-cyclodextrin. By studying the root mean square fluctuation (RMSF), it was revealed that the drugs were flexible in interaction with β-cyclodextrin; in contrast, β-cyclodextrin retained its rigidity in all simulated systems.
中文翻译:

激酶抑制剂与β-环糊精结合的详细计算研究:包合物形成
众所周知,某些药物的水溶性有限,通常会降低其对靶标的生物利用度。β-环糊精与药物形成包涵体复合物是改善药物递送的最佳方法之一。进行了广泛的微观分子动力学模拟,以研究β-环糊精与一类激酶抑制剂药物之间的相互作用。溶剂自由能的计算表明,这些药物不溶于水,并增加了对增加溶解度的载体的需求。通过计算结合自由能来评估这些药物与β-环糊精的结合,发现所有药物倾向于热力学结合。动力学特性研究表明,这些药物已装入β-环糊精中,因此,由于将药物加载到β-环糊精中,所有药物的均方位移(MSD)均急剧下降。药物的装载伴随着水从β-环糊精腔的中心排出。这些药物显示出不同的方向和加载到β-环糊精中的机制。通过研究均方根波动(RMSF),发现该药物与β-环糊精相互作用具有柔性。相反,β-环糊精在所有模拟系统中均保持其刚性。揭示了该药物与β-环糊精相互作用具有柔性。相反,β-环糊精在所有模拟系统中均保持其刚性。揭示了该药物与β-环糊精相互作用具有柔性。相反,β-环糊精在所有模拟系统中均保持其刚性。
更新日期:2020-12-09
中文翻译:

激酶抑制剂与β-环糊精结合的详细计算研究:包合物形成
众所周知,某些药物的水溶性有限,通常会降低其对靶标的生物利用度。β-环糊精与药物形成包涵体复合物是改善药物递送的最佳方法之一。进行了广泛的微观分子动力学模拟,以研究β-环糊精与一类激酶抑制剂药物之间的相互作用。溶剂自由能的计算表明,这些药物不溶于水,并增加了对增加溶解度的载体的需求。通过计算结合自由能来评估这些药物与β-环糊精的结合,发现所有药物倾向于热力学结合。动力学特性研究表明,这些药物已装入β-环糊精中,因此,由于将药物加载到β-环糊精中,所有药物的均方位移(MSD)均急剧下降。药物的装载伴随着水从β-环糊精腔的中心排出。这些药物显示出不同的方向和加载到β-环糊精中的机制。通过研究均方根波动(RMSF),发现该药物与β-环糊精相互作用具有柔性。相反,β-环糊精在所有模拟系统中均保持其刚性。揭示了该药物与β-环糊精相互作用具有柔性。相反,β-环糊精在所有模拟系统中均保持其刚性。揭示了该药物与β-环糊精相互作用具有柔性。相反,β-环糊精在所有模拟系统中均保持其刚性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号