当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coupled effects of Mn(II), pH and anionic ligands on the reactivity of nanostructured birnessite
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-11-20 , DOI: 10.1039/d0en01046d Qinzhi Li 1, 2, 3, 4, 5 , Rasesh Pokharel 1, 2, 3, 4, 5 , Lian Zhou 1, 2, 3, 4, 5 , Mathieu Pasturel 2, 5, 6, 7, 8 , Khalil Hanna 1, 2, 3, 4, 5
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2020-11-20 , DOI: 10.1039/d0en01046d Qinzhi Li 1, 2, 3, 4, 5 , Rasesh Pokharel 1, 2, 3, 4, 5 , Lian Zhou 1, 2, 3, 4, 5 , Mathieu Pasturel 2, 5, 6, 7, 8 , Khalil Hanna 1, 2, 3, 4, 5
Affiliation
|
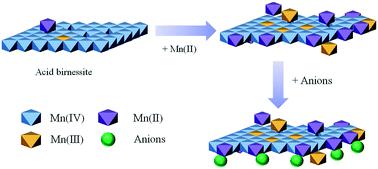
While the oxidative capacity of nanostructured birnessite-type manganese oxide has been widely investigated, no comprehensive work exists on the combined effects of dissolved Mn(II), pH and inorganic anions on sorption and redox reactions of organic contaminants with MnO2. Herein, we have showed how molecular interactions of two contrasting organic contaminants, pipemidic acid (PIP) and bisphenol A (BPA), with MnO2 surfaces controlled the removal kinetic behavior, which depended on contaminant type and coexisting anions. Competition between the contaminant and Mn(II) for binding at the edge sites determined the initial kinetic step, while buildup of Mn(II) at both edge and vacancy sites continuously decreased adsorption and subsequent oxidation over time. Redox interactions of Mn(II) with MnO2 surfaces was a pH-dependent process, and high pH favored Mn(II) removal and the comproportionation reaction, thus decreasing the adsorption and oxidation processes. At low Mn(II)/MnO2 ratio, MnO2 adsorbed more effectively anions such as phosphate or silicate, thus reducing interactions with organic compounds. These results highlight the combined suppressive effects of Mn(II), pH and naturally occurring anions on the reactivity of nanostructured birnessite and have strong implications on the fate of organic contaminants in terrestrial and aquatic environments.
中文翻译:

Mn(II),pH和阴离子配体对纳米结构水钠锰矿反应性的耦合作用
虽然已经广泛研究了纳米结构水钠锰矿型氧化锰的氧化能力,但尚无关于溶解的Mn(II),pH和无机阴离子对有机污染物与MnO 2的吸附和氧化还原反应的综合影响的综合研究。在这里,我们已经显示了两种相反的有机污染物,哌啶酸(PIP)和双酚A(BPA)与MnO 2表面的分子相互作用如何控制去除动力学行为,这取决于污染物类型和共存阴离子。污染物与Mn(II)之间在边缘部位的结合竞争决定了初始动力学步骤,而Mn(II)的积累)在边缘和空位处都随着时间的推移不断降低吸附和随后的氧化。Mn(II)与MnO 2表面的氧化还原相互作用是pH依赖的过程,高pH值有利于Mn(II)的去除和平衡反应,从而减少了吸附和氧化过程。在低Mn(II)/ MnO 2比率下,MnO 2可以更有效地吸附阴离子,例如磷酸根或硅酸根,从而减少与有机化合物的相互作用。这些结果突出了Mn(II),pH和天然阴离子对纳米结构水钠锰矿的反应性,并且对陆生和水生环境中有机污染物的命运具有重大影响。
更新日期:2020-12-09
中文翻译:

Mn(II),pH和阴离子配体对纳米结构水钠锰矿反应性的耦合作用
虽然已经广泛研究了纳米结构水钠锰矿型氧化锰的氧化能力,但尚无关于溶解的Mn(II),pH和无机阴离子对有机污染物与MnO 2的吸附和氧化还原反应的综合影响的综合研究。在这里,我们已经显示了两种相反的有机污染物,哌啶酸(PIP)和双酚A(BPA)与MnO 2表面的分子相互作用如何控制去除动力学行为,这取决于污染物类型和共存阴离子。污染物与Mn(II)之间在边缘部位的结合竞争决定了初始动力学步骤,而Mn(II)的积累)在边缘和空位处都随着时间的推移不断降低吸附和随后的氧化。Mn(II)与MnO 2表面的氧化还原相互作用是pH依赖的过程,高pH值有利于Mn(II)的去除和平衡反应,从而减少了吸附和氧化过程。在低Mn(II)/ MnO 2比率下,MnO 2可以更有效地吸附阴离子,例如磷酸根或硅酸根,从而减少与有机化合物的相互作用。这些结果突出了Mn(II),pH和天然阴离子对纳米结构水钠锰矿的反应性,并且对陆生和水生环境中有机污染物的命运具有重大影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号