当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mean Activity Coefficients of NaBr in Ternary System NaBr–Na2SO4–H2O Determined by the Cell Potential Method at 308.15 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-12-09 , DOI: 10.1021/acs.jced.0c00662 Yan Yu Song 1 , Shi-Hua Sang 1, 2 , Qi Ge 1 , Xing Liu 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-12-09 , DOI: 10.1021/acs.jced.0c00662 Yan Yu Song 1 , Shi-Hua Sang 1, 2 , Qi Ge 1 , Xing Liu 1
Affiliation
|
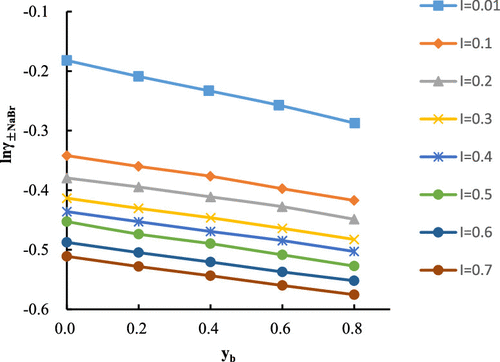
In this paper, the liquid-free battery Na-ISE | NaBr (m1), Na2SO4 (m2) |Br-ISE was used to measure the average activity coefficient of the ternary system NaBr–Na2SO4–H2O at 308.15 K by using the cell potential method. The ionic strength in the mixed solution is 0.01−0.70 mol·kg–1with different ionic strength fractions of Na2SO4, that is, yb = (0, 0.2, 0.4, 0.6, and 0.8). According to the Nernst equation, we can obtain the standard electromotive force E0 (263.52), electrode response slope (26.4), and linear correlation coefficient R2 (0.9998). These data indicate that the electrode response of the electrode pair is very good. On this basis, we used the Nernst equation to calculate the average activity coefficient of NaBr at 308.15 K in the NaBr–Na2SO4–H2O system. Finally, we got the corresponding mixed ion interaction parameters θBr,SO4, ψNa,Br,SO4 fitted by multilinear regression. In addition, the permeability coefficient, water activity, excess Gibbs free energy, and activity coefficient of Na2SO4 can also be calculated by using the Pitzer equation.
中文翻译:

在308.15 K下,通过细胞电势法测定三元体系NaBr–Na 2 SO 4 –H 2 O中NaBr的平均活度系数
本文中的无液电池 NaBr(m 1),Na 2 SO 4(m 2)| Br-ISE采用细胞电势法测量了308.15 K下三元体系NaBr–Na 2 SO 4 –H 2 O的平均活度系数。混合溶液中的离子强度为0.01−0.70 mol·kg –1,Na 2 SO 4的离子强度分数不同,即y b =(0、0.2、0.4、0.6和0.8)。根据能斯特方程,我们可以获得标准电动势E 0(263.52),电极响应斜率(26.4)和线性相关系数R 2(0.9998)。这些数据表明电极对的电极响应非常好。在此基础上,我们使用能斯特方程计算了NaBr–Na 2 SO 4 –H 2 O系统中308.15 K时NaBr的平均活度系数。最后,通过多元线性回归拟合得到相应的混合离子相互作用参数θBr,SO4,ψNa ,Br,SO4。另外,还可以通过使用Pitzer方程来计算渗透率系数,水活度,过量的吉布斯自由能以及Na 2 SO 4的活度系数。
更新日期:2021-01-14
中文翻译:

在308.15 K下,通过细胞电势法测定三元体系NaBr–Na 2 SO 4 –H 2 O中NaBr的平均活度系数
本文中的无液电池 NaBr(m 1),Na 2 SO 4(m 2)| Br-ISE采用细胞电势法测量了308.15 K下三元体系NaBr–Na 2 SO 4 –H 2 O的平均活度系数。混合溶液中的离子强度为0.01−0.70 mol·kg –1,Na 2 SO 4的离子强度分数不同,即y b =(0、0.2、0.4、0.6和0.8)。根据能斯特方程,我们可以获得标准电动势E 0(263.52),电极响应斜率(26.4)和线性相关系数R 2(0.9998)。这些数据表明电极对的电极响应非常好。在此基础上,我们使用能斯特方程计算了NaBr–Na 2 SO 4 –H 2 O系统中308.15 K时NaBr的平均活度系数。最后,通过多元线性回归拟合得到相应的混合离子相互作用参数θBr,SO4,ψNa ,Br,SO4。另外,还可以通过使用Pitzer方程来计算渗透率系数,水活度,过量的吉布斯自由能以及Na 2 SO 4的活度系数。











































 京公网安备 11010802027423号
京公网安备 11010802027423号