当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Semisynthesis of Human Ribonuclease–S
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-12-09 , DOI: 10.1021/acs.bioconjchem.0c00557 Jessica Sayers 1 , Evans C Wralstad 1 , Ronald T Raines 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-12-09 , DOI: 10.1021/acs.bioconjchem.0c00557 Jessica Sayers 1 , Evans C Wralstad 1 , Ronald T Raines 1
Affiliation
|
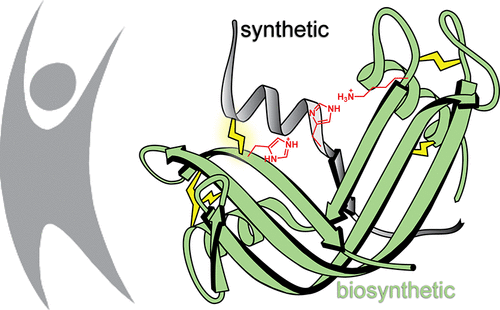
Since its conception, the ribonuclease S complex (RNase S) has led to historic discoveries in protein chemistry, enzymology, and related fields. Derived by the proteolytic cleavage of a single peptide bond in bovine pancreatic ribonuclease (RNase A), RNase S serves as a convenient and reliable model system for incorporating unlimited functionality into an enzyme. Applications of the RNase S system in biomedicine and biotechnology have, however, been hindered by two shortcomings: (1) the bovine-derived enzyme could elicit an immune response in humans, and (2) the complex is susceptible to dissociation. Here, we have addressed both limitations in the first semisynthesis of an RNase S conjugate derived from human pancreatic ribonuclease and stabilized by a covalent interfragment cross-link. We anticipate that this strategy will enable unprecedented applications of the “RNase–S” system.
中文翻译:

人核糖核酸酶-S的半合成
自诞生以来,核糖核酸酶 S 复合物 (RNase S) 在蛋白质化学、酶学和相关领域取得了历史性的发现。RNase S 源自牛胰核糖核酸酶 (RNase A) 中单个肽键的蛋白水解裂解,可用作将无限功能整合到酶中的方便可靠的模型系统。然而,RNase S 系统在生物医学和生物技术中的应用受到两个缺点的阻碍:(1) 牛源性酶可以引发人体免疫反应,(2) 该复合物容易解离。在这里,我们在源自人类胰腺核糖核酸酶并通过共价片段间交联稳定的 RNase S 缀合物的第一次半合成中存在两个局限性。
更新日期:2021-01-20
中文翻译:

人核糖核酸酶-S的半合成
自诞生以来,核糖核酸酶 S 复合物 (RNase S) 在蛋白质化学、酶学和相关领域取得了历史性的发现。RNase S 源自牛胰核糖核酸酶 (RNase A) 中单个肽键的蛋白水解裂解,可用作将无限功能整合到酶中的方便可靠的模型系统。然而,RNase S 系统在生物医学和生物技术中的应用受到两个缺点的阻碍:(1) 牛源性酶可以引发人体免疫反应,(2) 该复合物容易解离。在这里,我们在源自人类胰腺核糖核酸酶并通过共价片段间交联稳定的 RNase S 缀合物的第一次半合成中存在两个局限性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号