当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into Pore Formation by an α-Pore Forming Toxin: Protein and Lipid Bilayer Interactions of Cytolysin A
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-12-08 , DOI: 10.1021/acs.accounts.0c00551 Pradeep Sathyanarayana 1 , Sandhya S Visweswariah 1, 2 , K Ganapathy Ayappa 1, 3
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-12-08 , DOI: 10.1021/acs.accounts.0c00551 Pradeep Sathyanarayana 1 , Sandhya S Visweswariah 1, 2 , K Ganapathy Ayappa 1, 3
Affiliation
|
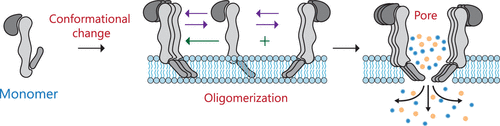
Pore forming toxins (PFTs) are the largest class of bacterial toxins playing a central role in bacterial pathogenesis. They are proteins specifically designed to form nanochannels in the membranes of target cells, ultimately resulting in cell death and establishing infection. PFTs are broadly classified as α- and β-PFTs, depending on secondary structures that form the transmembrane channel. A unique feature about this class of proteins is the drastic conformational changes and complex oligomerization pathways that occur upon exposure to the plasma membrane. A molecular understanding of pore formation has implications in designing novel intervention strategies to combat rising antimicrobial resistance, targeted-cancer therapy, as well as designing nanopores for specialized technologies. Central to unraveling the pore formation pathway is the availability of high resolution crystal structures. In this regard, β-toxins are better understood, when compared with α-toxins whose pore forming mechanisms are complicated by an incomplete knowledge of the driving forces for amphiphatic membrane-inserted helices to organize into functional pores. With the publication of the first crystal structure for an α-toxin, cytolysin A (ClyA), in 2009 we embarked on an extensive multiscale study to unravel its pore forming mechanism. This Account represents the collective mechanistic knowledge gained in our laboratories using a variety of experimental and theoretical techniques which include large scale molecular dynamics (MD) simulations, kinetic modeling studies, single-molecule fluorescence imaging, and super-resolution spectroscopy. We reported MD simulations of the ClyA protomer, oligomeric intermediates, and full pore complex in a lipid bilayer and mapped the conformational transitions that accompany membrane binding. Using single-molecule fluorescence imaging, the conformational transition was experimentally verified by analysis of various diffusion states of membrane bound ClyA. Importantly, we have uncovered a hitherto unknown putative cholesterol binding motif in the membrane-inserted helix of ClyA. Distinct binding pockets for cholesterol formed by adjacent membrane-inserted helices are revealed in MD simulations. Cholesterol appears to play a dual role by stabilizing both the membrane-inserted protomer as well as oligomeric intermediates. Molecular dynamics simulations and kinetic modeling studies suggest that the membrane-inserted arcs oligomerize reversibly to form the predominant transmembrane oligomeric intermediates during pore formation. We posit that this mechanistic understanding of the complex action of α-PFTs has implications in unraveling pore assembly across the wider family of bacterial toxins. With emerging antimicrobial resistance, alternate therapies may rely on disrupting pore functionality or oligomerization of these pathogenic determinants utilized by bacteria, and our study includes assessing the potential for dendrimers as pore blockers.
中文翻译:

通过α孔形成毒素的孔形成机理的见解:细胞溶素A的蛋白质和脂质双层相互作用。
毛孔形成毒素(PFT)是最大的一类细菌毒素,在细菌的发病机理中起着重要的作用。它们是专门设计用于在靶细胞膜上形成纳米通道的蛋白质,最终导致细胞死亡并感染。根据形成跨膜通道的二级结构,PFTs大致分为α-和β-PFTs。这类蛋白质的独特功能是在暴露于质膜时发生剧烈的构象变化和复杂的寡聚途径。对孔形成的分子理解对设计新的干预策略以对抗不断增长的抗微生物药耐药性,靶向癌症治疗以及为专门技术设计纳米孔具有重要意义。揭示孔形成途径的核心是高分辨率晶体结构的可用性。在这一点上,与α-毒素相比,其α-毒素的成孔机理由于对两亲性膜插入的螺旋组织成功能性孔的驱动力的不完全了解而变得更加容易理解。随着第一种α毒素晶体结构的溶细胞素A(ClyA)的发表,2009年,我们着手进行了广泛的多尺度研究,以阐明其孔形成机理。此帐户代表了我们在实验室中使用各种实验和理论技术获得的集体机械知识,其中包括大规模分子动力学(MD)模拟,动力学建模研究,单分子荧光成像和超分辨率光谱学。我们报道了脂质双分子层中ClyA启动子,寡聚中间体和全孔复合物的MD模拟,并绘制了与膜结合相关的构象转变。使用单分子荧光成像,通过分析膜结合的ClyA的各种扩散状态,通过实验验证了构象转变。重要的是,我们在膜插入的ClyA螺旋中发现了迄今未知的推定胆固醇结合基序。MD模拟显示了由相邻的膜插入的螺旋形成的胆固醇的独特结合口袋。胆固醇似乎通过稳定插入膜的前体以及寡聚中间体而起双重作用。分子动力学模拟和动力学建模研究表明,在孔形成过程中,插入膜的电弧可逆地低聚,从而形成主要的跨膜低聚中间体。我们认为,对α-PFTs复杂作用的这种机械理解对更广泛的细菌毒素家族的孔组装产生影响。随着新出现的抗药性,替代疗法可能依赖于破坏细菌利用的这些致病决定簇的孔功能或寡聚化,我们的研究包括评估树状聚合物作为孔阻断剂的潜力。我们认为,对α-PFTs复杂作用的这种机械理解对更广泛的细菌毒素家族的孔组装产生影响。随着新出现的抗药性,替代疗法可能依赖于破坏细菌利用的这些致病决定簇的孔功能或寡聚化,我们的研究包括评估树状聚合物作为孔阻断剂的潜力。我们认为,对α-PFTs复杂作用的这种机械理解对更广泛的细菌毒素家族的孔组装产生影响。随着新出现的抗药性,替代疗法可能依赖于破坏细菌利用的这些致病决定簇的孔功能或寡聚化,我们的研究包括评估树状聚合物作为孔阻断剂的潜力。
更新日期:2021-01-05
中文翻译:

通过α孔形成毒素的孔形成机理的见解:细胞溶素A的蛋白质和脂质双层相互作用。
毛孔形成毒素(PFT)是最大的一类细菌毒素,在细菌的发病机理中起着重要的作用。它们是专门设计用于在靶细胞膜上形成纳米通道的蛋白质,最终导致细胞死亡并感染。根据形成跨膜通道的二级结构,PFTs大致分为α-和β-PFTs。这类蛋白质的独特功能是在暴露于质膜时发生剧烈的构象变化和复杂的寡聚途径。对孔形成的分子理解对设计新的干预策略以对抗不断增长的抗微生物药耐药性,靶向癌症治疗以及为专门技术设计纳米孔具有重要意义。揭示孔形成途径的核心是高分辨率晶体结构的可用性。在这一点上,与α-毒素相比,其α-毒素的成孔机理由于对两亲性膜插入的螺旋组织成功能性孔的驱动力的不完全了解而变得更加容易理解。随着第一种α毒素晶体结构的溶细胞素A(ClyA)的发表,2009年,我们着手进行了广泛的多尺度研究,以阐明其孔形成机理。此帐户代表了我们在实验室中使用各种实验和理论技术获得的集体机械知识,其中包括大规模分子动力学(MD)模拟,动力学建模研究,单分子荧光成像和超分辨率光谱学。我们报道了脂质双分子层中ClyA启动子,寡聚中间体和全孔复合物的MD模拟,并绘制了与膜结合相关的构象转变。使用单分子荧光成像,通过分析膜结合的ClyA的各种扩散状态,通过实验验证了构象转变。重要的是,我们在膜插入的ClyA螺旋中发现了迄今未知的推定胆固醇结合基序。MD模拟显示了由相邻的膜插入的螺旋形成的胆固醇的独特结合口袋。胆固醇似乎通过稳定插入膜的前体以及寡聚中间体而起双重作用。分子动力学模拟和动力学建模研究表明,在孔形成过程中,插入膜的电弧可逆地低聚,从而形成主要的跨膜低聚中间体。我们认为,对α-PFTs复杂作用的这种机械理解对更广泛的细菌毒素家族的孔组装产生影响。随着新出现的抗药性,替代疗法可能依赖于破坏细菌利用的这些致病决定簇的孔功能或寡聚化,我们的研究包括评估树状聚合物作为孔阻断剂的潜力。我们认为,对α-PFTs复杂作用的这种机械理解对更广泛的细菌毒素家族的孔组装产生影响。随着新出现的抗药性,替代疗法可能依赖于破坏细菌利用的这些致病决定簇的孔功能或寡聚化,我们的研究包括评估树状聚合物作为孔阻断剂的潜力。我们认为,对α-PFTs复杂作用的这种机械理解对更广泛的细菌毒素家族的孔组装产生影响。随着新出现的抗药性,替代疗法可能依赖于破坏细菌利用的这些致病决定簇的孔功能或寡聚化,我们的研究包括评估树状聚合物作为孔阻断剂的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号