Cell Reports Physical Science ( IF 7.9 ) Pub Date : 2020-12-09 , DOI: 10.1016/j.xcrp.2020.100270 Hongjian He 1 , Xinyi Lin 1 , Difei Wu 1 , Jiaqing Wang 1 , Jiaqi Guo 1 , Douglas R Green 2 , Hongwei Zhang 3 , Bing Xu 1, 4
|
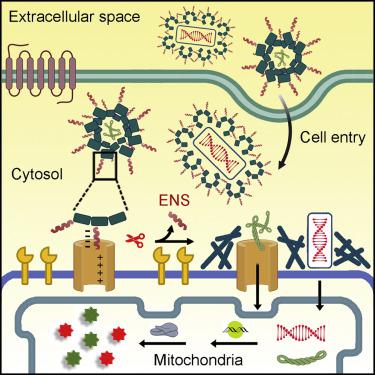
Since mitochondria contribute to tumorigenesis and drug resistance in cancer, mitochondrial genetic engineering promises a new direction for cancer therapy. Here, we report the use of the perimitochondrial enzymatic noncovalent synthesis (ENS) of peptides for delivering genes selectively into the mitochondria of cancer cells for mitochondrial genetic engineering. Specifically, the micelles of peptides bind to the voltage-dependent anion channel (VDAC) on mitochondria for the proteolysis by enterokinase (ENTK), generating perimitochondrial nanofibers in cancer cells. This process, facilitating selective delivery of nucleic acid or gene vectors into mitochondria of cancer cells, enables the mitochondrial transgene expression of CRISPR/Cas9, FUNDC1, p53, and fluorescent proteins. Mechanistic investigation indicates that the interaction of the peptide assemblies with the VDAC and mitochondrial membrane potential are necessary for mitochondria targeting. This local enzymatic control of intermolecular noncovalent interactions enables selective mitochondrial genetic engineering, thus providing a strategy for targeting cancer cells.
中文翻译:

用于癌细胞线粒体基因工程的酶促非共价合成
由于线粒体有助于癌症的肿瘤发生和耐药性,线粒体基因工程有望为癌症治疗开辟新方向。在这里,我们报告了使用肽的线粒体周酶促非共价合成 (ENS) 将基因选择性地递送到癌细胞的线粒体中以进行线粒体基因工程。具体来说,肽的胶束与线粒体上的电压依赖性阴离子通道 (VDAC) 结合,用于肠激酶 (ENTK) 的蛋白水解,在癌细胞中产生线粒体周围纳米纤维。这一过程促进了核酸或基因载体选择性递送到癌细胞的线粒体中,使 CRISPR/Cas9、FUNDC1、p53 和荧光蛋白的线粒体转基因表达成为可能。机理研究表明,肽组装体与 VDAC 和线粒体膜电位的相互作用是线粒体靶向所必需的。这种对分子间非共价相互作用的局部酶控制使选择性线粒体基因工程成为可能,从而为靶向癌细胞提供了一种策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号