当前位置:
X-MOL 学术
›
Polym. Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scalable, safer and greener syntheses of vinylimidazoles via reactive distillation of hydroxyethylimidazole intermediates
Polymer International ( IF 2.9 ) Pub Date : 2020-12-07 , DOI: 10.1002/pi.6161 Ali Alshaikh 1 , Kathryn E O'Harra 1 , Xiaoyang Liu 1 , John W Whitley 1 , Max S Mittenthal 1 , Wesley F Taylor 1 , C Heath Turner 1 , Jason E Bara 1
Polymer International ( IF 2.9 ) Pub Date : 2020-12-07 , DOI: 10.1002/pi.6161 Ali Alshaikh 1 , Kathryn E O'Harra 1 , Xiaoyang Liu 1 , John W Whitley 1 , Max S Mittenthal 1 , Wesley F Taylor 1 , C Heath Turner 1 , Jason E Bara 1
Affiliation
|
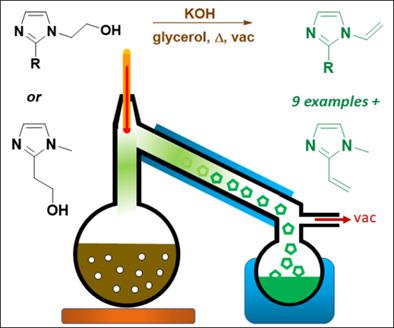
1‐Vinylimidazole has been extensively utilized by the polymer science community, due to its high reactivity for free radical polymerization and the variety of uses for both neutral polyvinylimidazole and cationic polyvinylimidazolium forms. While much rarer, 4‐vinylimidazoles and 2‐vinylimidazoles are less synthetically accessible. In comparison to conventional methods for the synthesis of vinylimidazole derivatives from energy‐intensive reaction conditions utilizing hazardous, gaseous precursors, herein we demonstrate a simple and versatile two‐step method applied to the synthesis of seven 1‐vinylimidazoles with different substituents as well as an initial demonstration of a facile method to synthesize the rare compound 1‐methyl‐2‐vinylimidazole. The process relies upon the synthesis of N‐hydroxyethylimidazole precursors via a ring‐opening reaction from substituted imidazoles with ethylene carbonate, a ‘green’ substance formed from CO2 and ethylene oxide. For the synthesis of 1‐methyl‐2‐vinylimidazole, the hydroxyethylimidazole intermediate is conveniently formed from 1,2‐dimethylimidazole and paraformaldehyde. These hydroxyethylimidazoles are subsequently dehydrated to the corresponding 1‐ or 2‐vinylimidazole forms using a base‐catalyzed reactive distillation. The optimization of process conditions is discussed, and properties of the vinylimidazole derivatives were computationally studied using density functional theory calculations. This work reveals scalable synthetic methods for previously inaccessible vinylimidazole compounds which can enable the design of new polymers. © 2020 Society of Chemical Industry
中文翻译:

通过羟乙基咪唑中间体的反应蒸馏可扩展,安全和绿色环保地合成乙烯基咪唑
1-Vinylimidazole因其对自由基聚合反应的高反应性以及中性聚乙烯基咪唑和阳离子聚乙烯基咪唑形式的多种用途而被聚合物科学界广泛使用。虽然数量少得多,但4-乙烯基咪唑和2-乙烯基咪唑在合成上较难获得。与使用危险的气态前体从能源密集型反应条件下合成乙烯基咪唑衍生物的常规方法相比,本文展示了一种简单而通用的两步法,该方法可用于合成七个具有不同取代基的1-乙烯基咪唑以及初步证明了一种合成稀有化合物1-甲基-2-乙烯基咪唑的简便方法。该过程依赖于N的合成-羟乙基咪唑前体通过取代的咪唑与碳酸亚乙酯的开环反应而形成,碳酸亚乙酯是由CO 2形成的“绿色”物质和环氧乙烷。对于1-甲基-2-乙烯基咪唑的合成,羟乙基咪唑中间体可以方便地由1,2-二甲基咪唑和低聚甲醛形成。随后使用碱催化反应蒸馏将这些羟乙基咪唑脱水成相应的1或2乙烯基咪唑形式。讨论了工艺条件的优化,并使用密度泛函理论计算对乙烯基咪唑衍生物的性质进行了计算研究。这项工作揭示了以前无法获得的乙烯基咪唑化合物的可扩展合成方法,这些方法可以设计新的聚合物。©2020化学工业协会
更新日期:2020-12-07
中文翻译:

通过羟乙基咪唑中间体的反应蒸馏可扩展,安全和绿色环保地合成乙烯基咪唑
1-Vinylimidazole因其对自由基聚合反应的高反应性以及中性聚乙烯基咪唑和阳离子聚乙烯基咪唑形式的多种用途而被聚合物科学界广泛使用。虽然数量少得多,但4-乙烯基咪唑和2-乙烯基咪唑在合成上较难获得。与使用危险的气态前体从能源密集型反应条件下合成乙烯基咪唑衍生物的常规方法相比,本文展示了一种简单而通用的两步法,该方法可用于合成七个具有不同取代基的1-乙烯基咪唑以及初步证明了一种合成稀有化合物1-甲基-2-乙烯基咪唑的简便方法。该过程依赖于N的合成-羟乙基咪唑前体通过取代的咪唑与碳酸亚乙酯的开环反应而形成,碳酸亚乙酯是由CO 2形成的“绿色”物质和环氧乙烷。对于1-甲基-2-乙烯基咪唑的合成,羟乙基咪唑中间体可以方便地由1,2-二甲基咪唑和低聚甲醛形成。随后使用碱催化反应蒸馏将这些羟乙基咪唑脱水成相应的1或2乙烯基咪唑形式。讨论了工艺条件的优化,并使用密度泛函理论计算对乙烯基咪唑衍生物的性质进行了计算研究。这项工作揭示了以前无法获得的乙烯基咪唑化合物的可扩展合成方法,这些方法可以设计新的聚合物。©2020化学工业协会











































 京公网安备 11010802027423号
京公网安备 11010802027423号