当前位置:
X-MOL 学术
›
Genes Cells
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
G‐quadruplex‐forming nucleic acids interact with splicing factor 3B subunit 2 and suppress innate immune gene expression
Genes to Cells ( IF 1.3 ) Pub Date : 2020-12-08 , DOI: 10.1111/gtc.12824 Kyoko Matsumoto 1, 2 , Keiji Okamoto 1 , Sachiko Okabe 1 , Risa Fujii 3 , Koji Ueda 3 , Kenichi Ohashi 2, 4 , Hiroyuki Seimiya 1, 2
Genes to Cells ( IF 1.3 ) Pub Date : 2020-12-08 , DOI: 10.1111/gtc.12824 Kyoko Matsumoto 1, 2 , Keiji Okamoto 1 , Sachiko Okabe 1 , Risa Fujii 3 , Koji Ueda 3 , Kenichi Ohashi 2, 4 , Hiroyuki Seimiya 1, 2
Affiliation
|
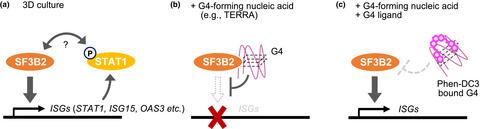
G‐quadruplex (G4), a non‐canonical higher‐order structure formed by guanine‐rich nucleic acid sequences, affects various genetic events in cis, including replication, transcription and translation. Whereas up‐regulation of innate immune/interferon‐stimulated genes (ISGs) is implicated in cancer progression, G4‐forming oligonucleotides that mimic telomeric repeat‐containing RNA suppress ISG induction in three‐dimensional (3D) culture of cancer cells. However, it is unclear how G4 suppresses ISG expression in trans. In this study, we found that G4 binding to splicing factor 3B subunit 2 (SF3B2) down‐regulated STAT1 phosphorylation and ISG expression in 3D‐cultured cancer cells. Liquid chromatography‐tandem mass spectrometry analysis identified SF3B2 as a G4‐binding protein. Either G4‐forming oligonucleotides or SF3B2 knockdown suppressed ISG induction, whereas Phen‐DC3, a G4‐stabilizing compound, reversed the inhibitory effect of G4‐forming oligonucleotides on ISG induction. Phen‐DC3 inhibited SF3B2 binding to G4 in vitro. SF3B2‐mediated ISG induction appeared to occur independently of RNA splicing because SF3B2 knockdown did not affect pre‐mRNA splicing under the experimental conditions, and pharmacological inhibition of splicing by pladienolide B did not repress ISG induction. These observations suggest that G4 disrupts the ability of SF3B2 to induce ISGs in cancer. We propose a new mode for gene regulation, which employs G4 as an inhibitory trans‐element.
中文翻译:

G-四链体形成核酸与剪接因子3B亚基2相互作用并抑制先天免疫基因表达
G-四链体(G4)是富含鸟嘌呤的核酸序列形成的非规范的高阶结构,它影响顺式的多种遗传事件,包括复制,转录和翻译。尽管先天免疫/干扰素刺激基因(ISG)的上调与癌症进展有关,但模仿G3形成的端粒重复序列RNA的寡核苷酸会抑制癌细胞在三维(3D)培养中的ISG诱导。但是,尚不清楚G4如何抑制反式中的ISG表达。在这项研究中,我们发现G4与剪接因子3B亚基2(SF3B2)的结合下调了3D培养癌细胞中STAT1的磷酸化和ISG表达。液相色谱-串联质谱分析确定SF3B2为G4结合蛋白。形成G4的寡核苷酸或SF3B2敲低均可抑制ISG诱导,而稳定G4的化合物Phen-DC3可逆转形成G4的寡核苷酸对ISG诱导的抑制作用。Phen-DC3在体外抑制SF3B2与G4的结合。SF3B2介导的ISG诱导似乎独立于RNA剪接而发生,因为在实验条件下SF3B2敲低并不影响pre-mRNA剪接,而普拉二烯内酯B对剪接的药理学抑制也不能抑制ISG诱导。这些观察结果表明G4破坏了SF3B2在癌症中诱导ISG的能力。我们提出了一种基因调控的新模式,该模式采用G4作为抑制性转座元素。
更新日期:2021-02-05
中文翻译:

G-四链体形成核酸与剪接因子3B亚基2相互作用并抑制先天免疫基因表达
G-四链体(G4)是富含鸟嘌呤的核酸序列形成的非规范的高阶结构,它影响顺式的多种遗传事件,包括复制,转录和翻译。尽管先天免疫/干扰素刺激基因(ISG)的上调与癌症进展有关,但模仿G3形成的端粒重复序列RNA的寡核苷酸会抑制癌细胞在三维(3D)培养中的ISG诱导。但是,尚不清楚G4如何抑制反式中的ISG表达。在这项研究中,我们发现G4与剪接因子3B亚基2(SF3B2)的结合下调了3D培养癌细胞中STAT1的磷酸化和ISG表达。液相色谱-串联质谱分析确定SF3B2为G4结合蛋白。形成G4的寡核苷酸或SF3B2敲低均可抑制ISG诱导,而稳定G4的化合物Phen-DC3可逆转形成G4的寡核苷酸对ISG诱导的抑制作用。Phen-DC3在体外抑制SF3B2与G4的结合。SF3B2介导的ISG诱导似乎独立于RNA剪接而发生,因为在实验条件下SF3B2敲低并不影响pre-mRNA剪接,而普拉二烯内酯B对剪接的药理学抑制也不能抑制ISG诱导。这些观察结果表明G4破坏了SF3B2在癌症中诱导ISG的能力。我们提出了一种基因调控的新模式,该模式采用G4作为抑制性转座元素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号