当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct C(sp3)−H Sulfonylation and Sulfuration Reactions of Isoquinoline‐1,3(2H,4H)‐diones under Metal‐free Conditions
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-12-07 , DOI: 10.1002/ajoc.202000668 Xing‐Lan Wang 1 , Xue Bai 2 , Chun‐Feng Wu 1 , Yong‐Xi Dong 1 , Min Zhang 1 , Ling‐Ling Fan 1 , Lei Tang 1 , Yuan‐Yong Yang 1 , Ji‐Quan Zhang 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-12-07 , DOI: 10.1002/ajoc.202000668 Xing‐Lan Wang 1 , Xue Bai 2 , Chun‐Feng Wu 1 , Yong‐Xi Dong 1 , Min Zhang 1 , Ling‐Ling Fan 1 , Lei Tang 1 , Yuan‐Yong Yang 1 , Ji‐Quan Zhang 1
Affiliation
|
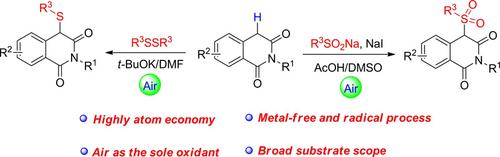
The first direct C(sp3)−H bond sulfonylation and sulfuration reactions of isoquinoline‐1,3‐(2H,4H)‐diones have been developed under transition‐metal‐free conditions. Using air as the sole oxidant, the C‐4 sulfonylated products of isoquinolones were synthesized using sulfinates as the sulfonating agents and NaI as an iodine source in AcOH/DMSO. The sulfuration reaction was realized for the first time using disulfides as sulfurating partners in a t‐BuOK/DMF catalytic system. Both procedures undergo a free radical reaction process with the advantages of high atom economy, green reaction conditions, readily accessible materials and broad substrate scope.
中文翻译:

在无金属条件下直接C(sp3)-H磺酰化和异喹啉-1,3(2H,4H)-二酮的硫化反应
在无过渡金属的条件下,首次开发了异喹啉-1,3-(2 H,4 H)-二酮的第一个直接C(sp 3)-H键磺酰化和硫化反应。使用空气作为唯一的氧化剂,使用亚磺酸盐作为磺化剂和NaI作为碘源在AcOH / DMSO中合成异喹诺酮的C-4磺酰化产物。硫化反应是在t- BuOK / DMF催化系统中首次使用二硫化物作为硫化伙伴实现的。两种方法都经过自由基反应过程,具有高原子经济性,绿色反应条件,易于获得的材料和广泛的底物范围的优点。
更新日期:2021-02-10
中文翻译:

在无金属条件下直接C(sp3)-H磺酰化和异喹啉-1,3(2H,4H)-二酮的硫化反应
在无过渡金属的条件下,首次开发了异喹啉-1,3-(2 H,4 H)-二酮的第一个直接C(sp 3)-H键磺酰化和硫化反应。使用空气作为唯一的氧化剂,使用亚磺酸盐作为磺化剂和NaI作为碘源在AcOH / DMSO中合成异喹诺酮的C-4磺酰化产物。硫化反应是在t- BuOK / DMF催化系统中首次使用二硫化物作为硫化伙伴实现的。两种方法都经过自由基反应过程,具有高原子经济性,绿色反应条件,易于获得的材料和广泛的底物范围的优点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号