Experimental Eye Research ( IF 3.0 ) Pub Date : 2020-12-08 , DOI: 10.1016/j.exer.2020.108391 Soo-Young Kim , Siva P. Kambhampati , Imran A. Bhutto , D. Scott McLeod , Gerard A. Lutty , Rangaramanujam M. Kannan
|
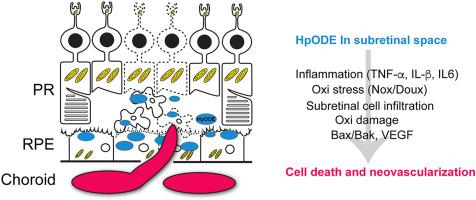
Oxidative stress, inflammation and neovascularization are the key pathological events that are implicated in human age-related macular degeneration (AMD). There are a limited number of animal models available for evaluating and developing new therapies. Most models represent late exudative or neovascular AMD (nAMD) but there is a relative paucity of models that mimic early events in AMD. The purpose of this study is to characterize the evolution of oxidative stress, inflammation, retinal degeneration and neovascularization in a rat model of AMD, created by subretinal injection of human lipid hydroperoxide (HpODE) that found in the sub-macular region in aged and AMD patients. Subretinal HpODE induced retinal pigment epithelium (RPE) and retinal degeneration resulting in loss of RPE cells, photoreceptors and retinal thinning. RPE degeneration and atrophy were detected by day 5, followed by neural tissue degeneration at day 12 with robust TUNEL positive cells. Western blot analysis confirmed an increase in pro-apoptotic Bak protein at day 12 in retinal tissues. Oxidative damage biomarkers (4-hydroxynonenal, malondialdehyde, 8-hydroxy-2′-deoxyguanosine, and nitrotyrosine) increased in retinal tissue from days 5–12. Müller glial activation was observed in the HpODE injected area at day 5 followed by its remodeling and migration in the outer retina by day 20. RT-qPCR analysis further indicated upregulation of pro-inflammatory genes (TNF-α, IL-1β and IL-6) both in retinal and RPE/choroidal tissue as early as day 2 and persisted until day 12. Upregulation of oxidative stress markers such as NADPH oxidase (NOX and DOUX family) was detected early in retinal tissue by day 2 followed by its upregulation in choroidal tissue at day 5. Neovascularization was demonstrated from day 12 to day 20 post HpODE injection in choroidal tissue. The results from this study indicate that subretinal HpODE induces advanced AMD phenotypes comprising many aspects of both dry/early and late) and neovascular/late AMD as observed in humans. Within 3 weeks via oxidative damage, upregulation of reactive oxygen species and pro-inflammatory genes, pro-apoptotic Bak and pro-angiogenic VEGF upregulation occurs leading to CNV formation. This experimental model of subretinal HpODE is an appropriate model for the study of AMD and provides an important platform for translational and basic research in developing new therapies particularly for early/dry AMD where currently no viable therapies are available.
中文翻译:

视网膜下脂质诱发的年龄相关性黄斑变性模型中脉络膜和视网膜中氧化应激,炎症和新生血管形成的演变
氧化应激,炎症和新生血管形成是与人类年龄相关的黄斑变性(AMD)涉及的关键病理事件。现有数量有限的动物模型可用于评估和开发新疗法。大多数模型代表晚期渗出性或新血管性AMD(nAMD),但相对缺乏模拟AMD早期事件的模型。这项研究的目的是表征在老年大鼠的黄斑区和视网膜下注射人类脂质氢过氧化物(HpODE)所产生的AMD大鼠模型中,氧化应激,炎症,视网膜变性和新血管形成的演变。耐心。视网膜下HpODE诱导视网膜色素上皮(RPE)和视网膜变性,导致RPE细胞,感光细胞和视网膜变薄。在第5天检测到RPE变性和萎缩,然后在第12天使用坚固的TUNEL阳性细胞进行神经组织变性。Western印迹分析证实了视网膜组织中促凋亡的Bak蛋白在第12天有所增加。从第5到12天,视网膜组织中的氧化损伤生物标志物(4-羟基壬烯醛,丙二醛,8-羟基-2'-脱氧鸟苷和硝基酪氨酸)增加。第5天,在注射HpODE的区域观察到Müller胶质细胞活化,然后在第20天观察到其重塑和迁移。RT-qPCR分析进一步表明促炎基因(TNF-α,IL-1β和IL- 6)最早在第2天就存在于视网膜和RPE /脉络膜组织中,并持续到第12天。到第2天,在视网膜组织中早期检测到氧化应激标记物(如NADPH氧化酶(NOX和DOUX家族))的上调,然后在第5天在脉络膜组织中上调。在向HpODE注射脉络膜组织后的第12天到第20天证实了新生血管形成。 。这项研究的结果表明,在人类中观察到,视网膜下HpODE诱导了晚期AMD表型,包括干/早,晚期和新血管/晚期AMD的许多方面。在3周内,通过氧化损伤,活性氧和促炎基因,促凋亡Bak和促血管生成VEGF的上调发生,导致CNV形成。










































 京公网安备 11010802027423号
京公网安备 11010802027423号