Mini-Reviews in Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-10-31 , DOI: 10.2174/1389557520666200611093510 Heba A. Elhady 1 , Hossa F. Al‑Shareef 2
|
Background and Objective: Due to the well-documented anti-proliferative activity of 2-thiohydantoin incorporated with pyrazole, oxadiazole, quinazoline, urea, β-naphthyl carbamate and Schiff bases, they are noteworthy in pharmaceutical chemistry.
Methods: An efficient approach for the synthesis of a novel series of 2-thiohydantoin derivatives incorporated with pyrazole and oxadiazole has proceeded via the reaction of the acyl hydrazide with chalcones and/or triethyl orthoformate. Schiff bases were synthesized by the reaction of the acyl hydrazide with different aromatic aldehydes. Moreover, Curtius rearrangement was applied to the acyl azide to obtain the urea derivative, quinazoline derivative, and carbamate derivative.
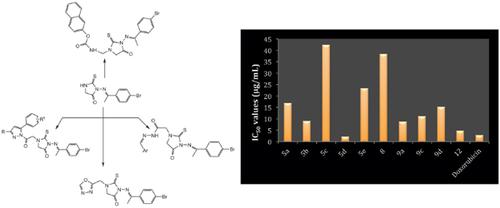
Results: The synthesized compounds structures were discussed and confirmed depending on their spectral data. The anticancer activity of these heterocyclic compounds was evaluated against the breast cancer cell line (MCF-7), where they showed variable activity. Compound 5d found to have a superior anticancer activity, where it has (IC50 = 2.07 ± 0.13 μg/mL) in comparison with the reference drug doxorubicin that has (IC50 = 2.79 ± 0.07 μg / mL). Then compound 5d subjected to further studies such as cell cycle analysis and apoptosis. Apoptosis was confirmed by the upregulation of Bax, downregulation of Bcl-2, and the increase of the caspase 3/7percentage.
Conclusion: Insertion of pyrazole, oxadiazole and, quinazoline moieties with 2-thiohydantoin moiety led to the enhancement of its anti-proliferative activity. Hence they can be used as anticancer agents.
中文翻译:

某些2-硫代乙内酰脲衍生物的合成,表征,抗增殖评价和DNA流式细胞术分析
背景与目的:由于2-硫代乙内酰脲与吡唑,恶二唑,喹唑啉,尿素,β-萘甲酸氨基甲酸酯和席夫碱结合的抗增殖活性已得到充分证明,因此在药物化学中值得关注。
方法:通过酰基酰肼与查耳酮和/或原甲酸三乙酯的反应,进行了一种合成一系列新的2-硫代乙内酰脲衍生物并掺入吡唑和恶二唑的有效方法。通过酰肼与不同的芳族醛的反应合成席夫碱。此外,对酰基叠氮化物进行Curtius重排,以获得脲衍生物,喹唑啉衍生物和氨基甲酸酯衍生物。
结果:讨论了合成的化合物结构,并根据其光谱数据进行了确认。评估了这些杂环化合物对乳腺癌细胞系(MCF-7)的抗癌活性,这些化合物表现出可变的活性。发现化合物5d具有较高的抗癌活性,与参考药物阿霉素(IC50 = 2.79±0.07μg/ mL)相比,其具有(IC50 = 2.07±0.13μg/ mL)。然后对化合物5d进行进一步研究,例如细胞周期分析和细胞凋亡。Bax的上调,Bcl-2的下调和caspase 3/7百分比的增加证实了细胞凋亡。
结论:插入带有2-硫代乙内酰脲部分的吡唑,恶二唑和喹唑啉部分可增强其抗增殖活性。因此它们可以用作抗癌剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号