Molecular Catalysis ( IF 3.9 ) Pub Date : 2020-12-07 , DOI: 10.1016/j.mcat.2020.111321 Alexander F. Schmidt , Anna A. Kurokhtina , Elizaveta V. Larina , Elena V. Vidyaeva , Nadezhda A. Lagoda
|
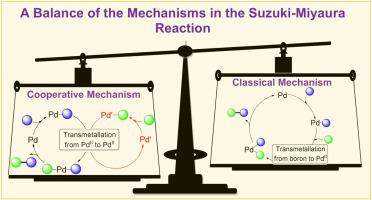
Herein, the independent differential selectivity in the Suzuki-Miyaura reaction of two competing arylboronic acids with regard to the nature of the aryl halide (both halide and aryl moieties) was established. This pattern cannot be realized via the “textbook” mechanism of the reaction, which suggests the interaction of boron-containing species with an ArPdX intermediate (product of the aryl halide catalytic activation). The data obtained indicate the participation of two distinct palladium species in the aryl halide and arylboronic acid activations in the catalytic cycle of biaryl formation, which are in accordance with the so-called cooperative mechanism.
中文翻译:

铃木-宫浦反应中的非经典合作机制–是否可能?
在此,关于芳基卤化物(卤化物和芳基部分)的性质,建立了两种竞争的芳基硼酸在Suzuki-Miyaura反应中的独立微分选择性。这种模式无法通过反应的“教科书”机制来实现,该机制表明含硼物质与ArPdX中间体(芳基卤化物催化活化的产物)之间的相互作用。获得的数据表明两种不同的钯物质参与联芳基形成的催化循环中的芳基卤化物和芳基硼酸活化,这与所谓的协同机理一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号