当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Different Hepatic Concentrations of Bromobenzene, 1,2-Dibromobenzene, and 1,4-Dibromobenzene in Humanized-Liver Mice Predicted Using Simplified Physiologically Based Pharmacokinetic Models as Putative Markers of Toxicological Potential
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-12-06 , DOI: 10.1021/acs.chemrestox.0c00387 Tomonori Miura 1 , Makiko Shimizu 1 , Shotaro Uehara 2 , Manae Yoshizawa 1 , Ayane Nakano 1 , Mayu Yanagi 1 , Yusuke Kamiya 1 , Norie Murayama 1 , Hiroshi Suemizu 2 , Hiroshi Yamazaki 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-12-06 , DOI: 10.1021/acs.chemrestox.0c00387 Tomonori Miura 1 , Makiko Shimizu 1 , Shotaro Uehara 2 , Manae Yoshizawa 1 , Ayane Nakano 1 , Mayu Yanagi 1 , Yusuke Kamiya 1 , Norie Murayama 1 , Hiroshi Suemizu 2 , Hiroshi Yamazaki 1
Affiliation
|
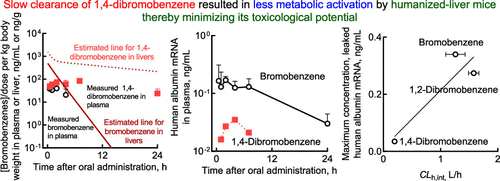
Bromobenzene is an industrial solvent that elicits toxicity predominantly in the liver. In this study, the hepatic concentrations of bromobenzene and its related compounds 1,2-dibromobenzene and 1,4-dibromobenzene in humanized-liver mice were predicted after single oral administrations by simplified physiologically based pharmacokinetic (PBPK) models that had been set up on experimental plasma concentrations after single oral doses of 100 mg/kg to rats and 100–250 mg/kg to control mice and humanized-liver mice. The output values by simplified PBPK models were consistent with measured blood substrate concentrations in rats, control mice, and humanized-liver mice with suitable input parameter values derived from in silico prediction and the literature or estimated by fitting the measured plasma substrate concentrations. The predicted time-dependent hepatic concentrations after virtual administrations in humanized-liver mice were partly confirmed with single measured hepatic concentrations of bromobenzene and 1,4-dibromobenzene 2 h after oral doses of 150–250 mg/kg to humanized-liver mice. Moreover, leaked human albumin mRNA, a marker of the extent of human hepatic injuries, in humanized-liver mouse plasma was detected after oral administration of bromobenzene, 1,2-dibromobenzene, and 1,4-dibromobenzene. These results suggest that dosimetry approaches for determining tissue and/or blood exposures of hepatic toxicants bromobenzene, 1,2-dibromobenzene, and 1,4-dibromobenzene in humanized-liver mice were useful after virtual oral doses using simplified PBPK models. Using simplified PBPK models and plasma data from humanized-liver mice has potential to predict and evaluate the hepatic toxicity of bromobenzenes and related compounds in humanized-liver mice and in humans.
中文翻译:

使用简化的基于生理学的药代动力学模型作为毒理学潜力的假定标志物预测人源化肝脏小鼠中溴苯、1,2-二溴苯和 1,4-二溴苯的不同肝脏浓度
溴苯是一种工业溶剂,主要在肝脏中引起毒性。在这项研究中,在人源化肝小鼠中,溴苯及其相关化合物 1,2-二溴苯和 1,4-二溴苯的肝脏浓度通过基于简化生理学的药代动力学 (PBPK) 模型在单次口服给药后进行预测大鼠单次口服 100 mg/kg 和对照小鼠和人源化肝小鼠单次口服 100-250 mg/kg 后的实验血浆浓度。简化的 PBPK 模型的输出值与大鼠、对照小鼠和人源化肝小鼠中测量的血液底物浓度一致,合适的输入参数值来自计算机预测和文献,或通过拟合测量的血浆底物浓度估计。人源化肝小鼠虚拟给药后预测的时间依赖性肝脏浓度部分通过在人源化肝小鼠口服 150–250 mg/kg 后 2 小时测量的溴苯和 1,4-二溴苯的单一肝脏浓度得到部分证实。此外,在口服溴苯、1,2-二溴苯和1,4-二溴苯后,在人源化肝脏小鼠血浆中检测到泄漏的人白蛋白mRNA,这是人类肝损伤程度的标志物。这些结果表明,在使用简化的 PBPK 模型进行虚拟口服剂量后,用于确定肝毒物溴苯、1,2-二溴苯和 1,4-二溴苯在人源化肝小鼠中的组织和/或血液暴露的剂量学方法是有用的。
更新日期:2020-12-21
中文翻译:

使用简化的基于生理学的药代动力学模型作为毒理学潜力的假定标志物预测人源化肝脏小鼠中溴苯、1,2-二溴苯和 1,4-二溴苯的不同肝脏浓度
溴苯是一种工业溶剂,主要在肝脏中引起毒性。在这项研究中,在人源化肝小鼠中,溴苯及其相关化合物 1,2-二溴苯和 1,4-二溴苯的肝脏浓度通过基于简化生理学的药代动力学 (PBPK) 模型在单次口服给药后进行预测大鼠单次口服 100 mg/kg 和对照小鼠和人源化肝小鼠单次口服 100-250 mg/kg 后的实验血浆浓度。简化的 PBPK 模型的输出值与大鼠、对照小鼠和人源化肝小鼠中测量的血液底物浓度一致,合适的输入参数值来自计算机预测和文献,或通过拟合测量的血浆底物浓度估计。人源化肝小鼠虚拟给药后预测的时间依赖性肝脏浓度部分通过在人源化肝小鼠口服 150–250 mg/kg 后 2 小时测量的溴苯和 1,4-二溴苯的单一肝脏浓度得到部分证实。此外,在口服溴苯、1,2-二溴苯和1,4-二溴苯后,在人源化肝脏小鼠血浆中检测到泄漏的人白蛋白mRNA,这是人类肝损伤程度的标志物。这些结果表明,在使用简化的 PBPK 模型进行虚拟口服剂量后,用于确定肝毒物溴苯、1,2-二溴苯和 1,4-二溴苯在人源化肝小鼠中的组织和/或血液暴露的剂量学方法是有用的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号