Cellular Signalling ( IF 4.4 ) Pub Date : 2020-12-05 , DOI: 10.1016/j.cellsig.2020.109870 Yuehong Wang 1 , Ruihuan Yu 1 , Lingyun Wu 2 , Guangdong Yang 1
|
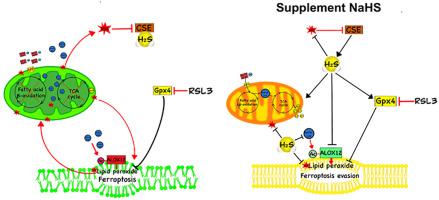
Recognized as a novel and important gasotransmitter, hydrogen sulfide (H2S) is widely present in various tissues and organs. Cystathionine gamma-lyase (CSE)-derived H2S has been shown to regulate oxidative stress and lipid metabolism. The aim of the present study is to examine the role of H2S in ferroptosis and lipid peroxidation in mouse myoblasts and skeletal muscles. Ferroptosis agonist RSL3 inhibited the expressions of Gpx4 and reduced CSE/H2S signaling, which lead to increased oxidative stress, lipid peroxidation, and ferroptotic cell death. In addition, ferroptosis antagonist ferrostatin-1 (Fer-1) up-regulated the expression of CSE, scavenged the generation of reactive oxygen species (ROS) and lipid peroxidation, and improved cell viability. Exogenously applied NaHS was also able to block RSL3-induced ferroptotic cell death. Neither RSL3 nor H2S affected cell apoptosis. Furthermore, H2S reversed RSL3-induced Drp1 expression and mitochondrial damage, which lead to abnormal lipid metabolism as evidenced by altered expressions of ACSL4, FAS, ACC and CPT1 as well as higher acetyl-CoA contents in both cytoplasm and mitochondria. RSL3 promoted the protein expression and acetylation of ALOX12, a key protein in initiating membrane phospholipid oxidation, while the addition of NaHS attenuated ALOX12 acetylation and protected from membrane lipid peroxidation. Moreover, we observed that CSE deficiency alters the expressions of ferroptosis and lipid peroxidation-related proteins and enhances global protein acetylation in mouse skeletal muscles under aging or injury conditions. These results indicate that downregulation of CSE/H2S signaling would contribute to mitochondrial damage, abnormal lipid metabolism, membrane lipid peroxidation, and ferroptotic cell death. CSE/H2S system can be a target for preventing ferroptosis in skeletal muscle.
中文翻译:

硫化氢通过抑制 ALOX12 乙酰化保护成肌细胞免于铁死亡
硫化氢 (H 2 S) 被认为是一种新型且重要的气体传递体,广泛存在于各种组织和器官中。胱硫醚 γ-裂解酶 (CSE) 衍生的 H 2 S 已被证明可以调节氧化应激和脂质代谢。本研究的目的是检查 H 2 S 在小鼠成肌细胞和骨骼肌铁死亡和脂质过氧化中的作用。Ferroptosis 激动剂 RSL3 抑制 Gpx4 的表达并降低 CSE/H 2S 信号,导致氧化应激增加、脂质过氧化和铁死亡细胞死亡。此外,ferroptosis拮抗剂ferrostatin-1(Fer-1)上调CSE的表达,清除活性氧(ROS)和脂质过氧化的产生,并提高细胞活力。外源性应用的 NaHS 也能够阻止 RSL3 诱导的铁死亡细胞死亡。RSL3 和H 2 S都不影响细胞凋亡。此外,H 2S 逆转了 RSL3 诱导的 Drp1 表达和线粒体损伤,这导致脂质代谢异常,如 ACSL4、FAS、ACC 和 CPT1 的表达改变以及细胞质和线粒体中更高的乙酰辅酶 A 含量证明了这一点。RSL3 促进了 ALOX12 的蛋白质表达和乙酰化,ALOX12 是启动膜磷脂氧化的关键蛋白质,而 NaHS 的加入减弱了 ALOX12 乙酰化并防止膜脂过氧化。此外,我们观察到 CSE 缺乏会改变铁死亡和脂质过氧化相关蛋白的表达,并在衰老或损伤条件下增强小鼠骨骼肌的整体蛋白乙酰化。这些结果表明 CSE/H 2 的下调S 信号会导致线粒体损伤、脂质代谢异常、膜脂过氧化和铁死亡。CSE/H 2 S 系统可以成为预防骨骼肌铁死亡的目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号