当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ni-Catalyzed Regioselective 1,2-Dialkylation of Alkenes Enabled by the Formation of Two C(sp3)–C(sp3) Bonds
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-12-03 , DOI: 10.1021/jacs.0c09778 Roshan K Dhungana 1 , Rishi R Sapkota 1 , Laura M Wickham 1 , Doleshwar Niroula 1 , Ramesh Giri 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-12-03 , DOI: 10.1021/jacs.0c09778 Roshan K Dhungana 1 , Rishi R Sapkota 1 , Laura M Wickham 1 , Doleshwar Niroula 1 , Ramesh Giri 1
Affiliation
|
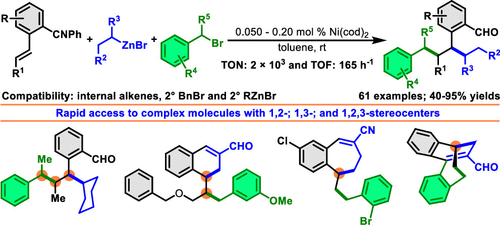
We disclose a Ni-catalyzed vicinal difunctionalization of alkenes with benzyl halides and alkylzinc reagents, which produces products with two new alkyl-alkyl bonds. This alkene dialkylation is effective in combining secondary benzyl halides and secondary alkylzinc reagents with internal alkenes, which furnishes products with three contiguous all-carbon secondary stereocenters. The products can be readily elaborated to access complex tetralene, benzosuberene, and bicyclodecene cores. The reaction also features as the most efficient alkene difunctionalization process to date with catalyst loadings down to 500 ppm and the catalytic turnover number (TON) and turnover frequency (TOF) registering up to 2 × 103 and 165 h-1 at rt, respectively.
中文翻译:

Ni 催化通过形成两个 C(sp3)–C(sp3) 键实现烯烃区域选择性 1,2-二烷基化
我们公开了一种镍催化的烯烃与卤化苄和烷基锌试剂的邻位双官能化反应,其产生具有两个新的烷基-烷基键的产物。这种烯烃二烷基化可有效地将仲苄基卤化物和仲烷基锌试剂与内部烯烃结合,从而提供具有三个连续的全碳仲立构中心的产物。该产品可以很容易地加工成复杂的四萘嵌苯、苯并环戊二烯和双环癸烯核心。该反应也是迄今为止最有效的烯烃双官能化工艺,催化剂负载量低至 500 ppm,室温下催化周转数 (TON) 和周转频率 (TOF) 分别高达 2 × 103 和 165 h-1。
更新日期:2020-12-03
中文翻译:

Ni 催化通过形成两个 C(sp3)–C(sp3) 键实现烯烃区域选择性 1,2-二烷基化
我们公开了一种镍催化的烯烃与卤化苄和烷基锌试剂的邻位双官能化反应,其产生具有两个新的烷基-烷基键的产物。这种烯烃二烷基化可有效地将仲苄基卤化物和仲烷基锌试剂与内部烯烃结合,从而提供具有三个连续的全碳仲立构中心的产物。该产品可以很容易地加工成复杂的四萘嵌苯、苯并环戊二烯和双环癸烯核心。该反应也是迄今为止最有效的烯烃双官能化工艺,催化剂负载量低至 500 ppm,室温下催化周转数 (TON) 和周转频率 (TOF) 分别高达 2 × 103 和 165 h-1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号