当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic Synthesis of α-Trifluoromethyl Allylboronic Acids by Enantioselective 1,2-Borotropic Migration
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-12-03 , DOI: 10.1021/jacs.0c09923 Sybrand J. T. Jonker 1 , Ramasamy Jayarajan 1 , Tautvydas Kireilis 1 , Marie Deliaval 1 , Lars Eriksson 2 , Kálmán J. Szabó 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-12-03 , DOI: 10.1021/jacs.0c09923 Sybrand J. T. Jonker 1 , Ramasamy Jayarajan 1 , Tautvydas Kireilis 1 , Marie Deliaval 1 , Lars Eriksson 2 , Kálmán J. Szabó 1
Affiliation
|
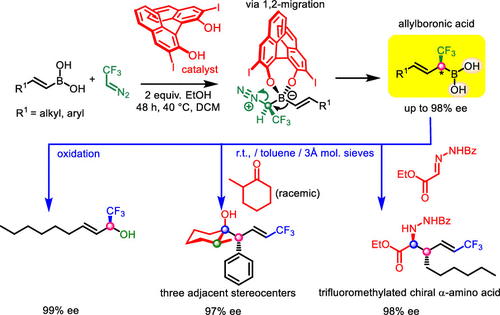
Chiral α-substituted allylboronic acids were synthesized by asymmetric homologation of alkenylboronic acids using CF3/TMS-diazomethanes in the presence of BINOL catalyst and ethanol. The chiral α-substituted allylboronic acids were reacted with aldehydes or oxidized to alcohols in situ with a high degree of chirality transfer. The oxygen-sensitive allylboronic acids can be purified via their isolated diaminonaphthalene (DanH)-protected derivatives. The highly reactive purified allylboronic acids reacted in a self-catalyzed reaction at room temperature with ketones, imines, and indoles to give congested trifluoromethylated homoallylic alcohols/amines with up to three contiguous stereocenters.
中文翻译:

对映选择性 1,2-硼迁移法有机催化合成 α-三氟甲基烯丙基硼酸
在 BINOL 催化剂和乙醇的存在下,使用 CF3/TMS-重氮甲烷,通过烯基硼酸的不对称同系化合成了手性 α-取代的烯丙基硼酸。手性α-取代的烯丙基硼酸与醛反应或原位氧化成醇,具有高度的手性转移。氧敏感的烯丙基硼酸可以通过其分离的二氨基萘 (DanH) 保护的衍生物进行纯化。高反应性纯化的烯丙基硼酸在室温下与酮、亚胺和吲哚在自催化反应中反应,得到具有多达三个连续立体中心的拥挤的三氟甲基化高烯丙醇/胺。
更新日期:2020-12-03
中文翻译:

对映选择性 1,2-硼迁移法有机催化合成 α-三氟甲基烯丙基硼酸
在 BINOL 催化剂和乙醇的存在下,使用 CF3/TMS-重氮甲烷,通过烯基硼酸的不对称同系化合成了手性 α-取代的烯丙基硼酸。手性α-取代的烯丙基硼酸与醛反应或原位氧化成醇,具有高度的手性转移。氧敏感的烯丙基硼酸可以通过其分离的二氨基萘 (DanH) 保护的衍生物进行纯化。高反应性纯化的烯丙基硼酸在室温下与酮、亚胺和吲哚在自催化反应中反应,得到具有多达三个连续立体中心的拥挤的三氟甲基化高烯丙醇/胺。











































 京公网安备 11010802027423号
京公网安备 11010802027423号