当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reactive Extraction of Monocarboxylic Acids (Formic, Acetic, and Propionic) Using Tributyl Phosphate in Green Solvents (Cyclopentyl Methyl Ether and 2-Methyltetrahydrofuran)
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-04 , DOI: 10.1021/acs.jced.0c00486 Feride Naime Türk 1 , Süheyla Çehreli 1 , Nilay Baylan 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-12-04 , DOI: 10.1021/acs.jced.0c00486 Feride Naime Türk 1 , Süheyla Çehreli 1 , Nilay Baylan 1
Affiliation
|
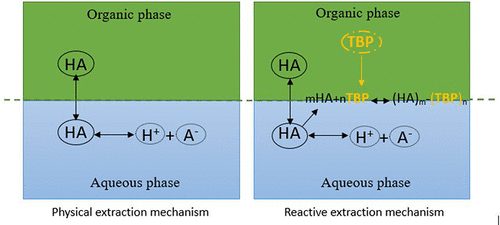
The separation of monocarboxylic acids (formic, acetic, and propionic) from aqueous solutions is still a current issue for both scientific and industrial fields. Reactive extraction with an adequate extractant and diluent system has been focused and researched as an alternative to other separation techniques. Lately, the use of green solvents instead of conventional organic solvents in carboxylic acid extraction has received a great amount of attention. In this aspect, in the present research, the recovery of formic acid (FA), acetic acid (AA), and propionic acid (PA) from aqueous solutions was carried out by reactive extraction with green solvents. In the current reactive study, tributyl phosphate (TBP) has been used as an extractant, and green solvents, cyclopentyl methyl ether (CPME) and 2-methyltetrahydrofuran (2-MeTHF), have been utilized as diluents. The effect of TBP concentration (0.7–5.2 mol·kg–1) on the extraction power was investigated. In addition, the effects of the use of two different green solvents on extraction were also investigated. Distribution coefficient (D), extraction efficiency (E%), and loading factor (Z) values were ascertained and interpreted. D, E, and Z were determined to be in the ranges of 0.289–4.003, 22.41–78.41%, and 0.198–2.218, respectively. It was also observed that, for the studied range, the extractant concentration was increased to be effective when using CPME as the diluent, but a similar effect was not seen when 2-MeTHF was used. However, 2-MeTHF was observed to be a more effective solvent for physical extraction. The extraction efficiency in both diluents increased in the order of PA > AA ≥ FA.
中文翻译:

在绿色溶剂(环戊基甲基醚和2-甲基四氢呋喃)中使用磷酸三丁酯反应性萃取一元羧酸(甲酸,乙酸和丙酸)
从水溶液中分离一元羧酸(甲酸,乙酸和丙酸)仍然是科学和工业领域的当前问题。使用适当的萃取剂和稀释剂系统进行反应性萃取已成为研究和替代其他分离技术的重点。近来,在羧酸萃取中使用绿色溶剂代替常规有机溶剂已引起广泛关注。在这一方面,在本研究中,通过与绿色溶剂反应萃取从水溶液中回收甲酸(FA),乙酸(AA)和丙酸(PA)。在目前的反应性研究中,磷酸三丁酯(TBP)已用作萃取剂,绿色溶剂环戊基甲基醚(CPME)和2-甲基四氢呋喃(2-MeTHF)已被用作稀释剂。TBP浓度的影响(0.7-5.2 mol·kg–1)对提取功率进行了研究。此外,还研究了使用两种不同绿色溶剂对萃取的影响。确定并解释了分配系数(D),提取效率(E%)和负载系数(Z)值。D,E和Z分别确定在0.289–4.003、22.41–78.41%和0.198–2.218的范围内。还观察到,在所研究的范围内,当使用CPME作为稀释剂时,萃取剂的浓度增加以有效,但是当使用2-MeTHF时未观察到类似的效果。然而,观察到2-MeTHF是更有效的物理提取溶剂。两种稀释剂的提取效率均按PA> AA≥FA的顺序增加。
更新日期:2021-01-14
中文翻译:

在绿色溶剂(环戊基甲基醚和2-甲基四氢呋喃)中使用磷酸三丁酯反应性萃取一元羧酸(甲酸,乙酸和丙酸)
从水溶液中分离一元羧酸(甲酸,乙酸和丙酸)仍然是科学和工业领域的当前问题。使用适当的萃取剂和稀释剂系统进行反应性萃取已成为研究和替代其他分离技术的重点。近来,在羧酸萃取中使用绿色溶剂代替常规有机溶剂已引起广泛关注。在这一方面,在本研究中,通过与绿色溶剂反应萃取从水溶液中回收甲酸(FA),乙酸(AA)和丙酸(PA)。在目前的反应性研究中,磷酸三丁酯(TBP)已用作萃取剂,绿色溶剂环戊基甲基醚(CPME)和2-甲基四氢呋喃(2-MeTHF)已被用作稀释剂。TBP浓度的影响(0.7-5.2 mol·kg–1)对提取功率进行了研究。此外,还研究了使用两种不同绿色溶剂对萃取的影响。确定并解释了分配系数(D),提取效率(E%)和负载系数(Z)值。D,E和Z分别确定在0.289–4.003、22.41–78.41%和0.198–2.218的范围内。还观察到,在所研究的范围内,当使用CPME作为稀释剂时,萃取剂的浓度增加以有效,但是当使用2-MeTHF时未观察到类似的效果。然而,观察到2-MeTHF是更有效的物理提取溶剂。两种稀释剂的提取效率均按PA> AA≥FA的顺序增加。



























 京公网安备 11010802027423号
京公网安备 11010802027423号