Synthesis ( IF 2.2 ) Pub Date : 2020-12-03 , DOI: 10.1055/s-0040-1706608 Hua Zhao 1 , Hongbin Zhai 2 , Peng Shen 1 , Yeting Guo 1 , Jian Wei 2 , Yufen Zhao 1
|
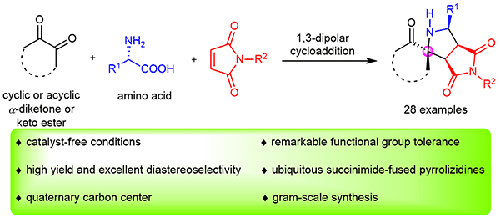
An efficient, one-pot, three-component [3+2] cycloaddition reaction of azomethine ylide obtained from α-dicarbonyl compounds (cyclic and acyclic diketone or keto ester) and amino acids with maleimides under catalyst-free conditions has been developed. This cascade protocol shows high efficiency and remarkable functional group tolerance, and the ubiquitous succinimide-fused pyrrolizidines with a highly compact and strained scaffold were obtained with high yield and excellent diastereoselectivity. Furthermore, this novel and atom-economical strategy could be performed on a gram scale with comparable reaction efficiency.
中文翻译:

通过α-二酮,氨基酸和马来酰亚胺的三组分反应直接合成琥珀酰亚胺基吡咯并核苷
在无催化剂条件下,开发了由α-二羰基化合物(环状和无环二酮或酮酯)和氨基酸与马来酰亚胺形成的高效,一锅,三组分的[3 + 2]环甲亚胺环加成反应。该级联方案显示出高效率和显着的官能团耐受性,并以高收率和优异的非对映选择性获得了具有高度致密和应变支架的无处不在的琥珀酰亚胺-融合的吡咯烷核苷。此外,这种新颖且原子经济的策略可以在克规模上以可比的反应效率进行。









































 京公网安备 11010802027423号
京公网安备 11010802027423号