当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational exploration of the 1,3‐dipolar cycloaddition reaction of 7‐isopropylidenebenzonorbornadiene with nitrile oxide and cyclic nitrone derivatives
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-12-03 , DOI: 10.1002/poc.4174 George Baffour Pipim 1 , Richard Tia 1 , Evans Adei 1
Journal of Physical Organic Chemistry ( IF 1.9 ) Pub Date : 2020-12-03 , DOI: 10.1002/poc.4174 George Baffour Pipim 1 , Richard Tia 1 , Evans Adei 1
Affiliation
|
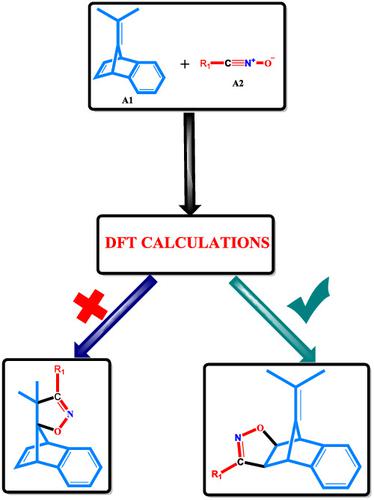
The synthesis of isoxazolidine and isoxazole derivatives, versatile building blocks for the construction of a wide range of complex heterocyclic architectures in synthetic organic and medicinal chemistry, is efficiently achieved via the 1,3‐dipolar cycloaddition reaction (1,3‐DC). Herein, we report an extensive theoretical study on the peri‐, regio‐, stereo, and enantio‐selectivities of 1,3‐DC of 7‐isopropylidenebenzonorbornadiene with nitrile oxide and cyclic nitrone derivatives using density functional theory calculations. Acetophenone‐substituted nitrile oxide periselectively adds across the endocyclic olefinic bond of the dipolarophile to furnish the exo‐cycloadduct as the major product, a reaction that has a rate constant of 1.88 × 109 s−1. The endo approach of this periselective path is the closest competing pathway with a rate constant of 4.59 × 107 s−1. Different substituents on the nitrile oxide do not affect the peri‐ and stereo‐selectivity of the reaction. Diethyl ether solvation has no substantial effect on the energetic patterns observed in the gas phase computation. Also, we report a novel 1,3‐DC between cyclic nitrone derivatives and 7‐isopropylidenebenzonorbornadiene as an efficient way to generate isoxazolidine derivatives. Even though the reactions of the cyclic nitrone derivatives have slightly higher activation barriers than the acyclic nitrile oxide derivatives, the former is more enantioselective than the latter. Whereas electron‐donating groups (EDGs) on the cyclic nitrone favor the formation of the exo‐cycloadduct, electron‐withdrawing groups (EWGs) favor the formation of the endo‐cycloadduct. Both 1,3‐dipoles add across the dipolarophile via a concerted asynchronous mechanism.
中文翻译:

7-异亚丙基苯并降冰片二烯与一氧化二氮和环硝酮衍生物的1,3-偶极环加成反应的计算探索
异恶唑烷和异恶唑衍生物的合成是通过1,3-偶极环加成反应(1,3-DC)有效实现的,可用于在合成有机和药物化学中构建各种复杂的杂环结构。本文中,我们使用密度泛函理论计算,对7-异亚丙基苯并降冰片二烯与一氧化二氮和环硝酮衍生物的1,3-DC的周,立构,立体和对映选择性进行了广泛的理论研究。苯乙酮取代的氧化腈横跨亲偶极的桥环烯键periselectively增加,得到的外-cycloadduct作为主要产物,其具有的1.88×10速率常数的反应9个小号-1。这种围选择性途径的内源途径是最接近的竞争途径,其速率常数为4.59×10 7 s -1。一氧化氮上的不同取代基不会影响反应的周向和立体选择性。乙醚溶剂化对气相计算中观察到的高能谱没有实质性影响。另外,我们报道了在环状硝酮衍生物和7-异亚丙基苯并降冰片二烯之间的新型1,3-DC化合物,它是产生异恶唑烷衍生物的有效方法。即使环状硝酮衍生物的反应具有比无环丁腈氧化物衍生物更高的活化势垒,但前者比后者具有更高的对映选择性。环状硝酮上的给电子基团(EDGs)有助于形成外-cycloadduct,吸电子基团(专家工作组)青睐的形成内切-cycloadduct。两个1,3-偶极子通过协调的异步机制跨亲极子增加。
更新日期:2020-12-03
中文翻译:

7-异亚丙基苯并降冰片二烯与一氧化二氮和环硝酮衍生物的1,3-偶极环加成反应的计算探索
异恶唑烷和异恶唑衍生物的合成是通过1,3-偶极环加成反应(1,3-DC)有效实现的,可用于在合成有机和药物化学中构建各种复杂的杂环结构。本文中,我们使用密度泛函理论计算,对7-异亚丙基苯并降冰片二烯与一氧化二氮和环硝酮衍生物的1,3-DC的周,立构,立体和对映选择性进行了广泛的理论研究。苯乙酮取代的氧化腈横跨亲偶极的桥环烯键periselectively增加,得到的外-cycloadduct作为主要产物,其具有的1.88×10速率常数的反应9个小号-1。这种围选择性途径的内源途径是最接近的竞争途径,其速率常数为4.59×10 7 s -1。一氧化氮上的不同取代基不会影响反应的周向和立体选择性。乙醚溶剂化对气相计算中观察到的高能谱没有实质性影响。另外,我们报道了在环状硝酮衍生物和7-异亚丙基苯并降冰片二烯之间的新型1,3-DC化合物,它是产生异恶唑烷衍生物的有效方法。即使环状硝酮衍生物的反应具有比无环丁腈氧化物衍生物更高的活化势垒,但前者比后者具有更高的对映选择性。环状硝酮上的给电子基团(EDGs)有助于形成外-cycloadduct,吸电子基团(专家工作组)青睐的形成内切-cycloadduct。两个1,3-偶极子通过协调的异步机制跨亲极子增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号