当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
ABCB1/MDR1/P‐gp employs an ATP‐dependent twist‐and‐squeeze mechanism to export hydrophobic drugs
FEBS Letters ( IF 3.5 ) Pub Date : 2020-12-11 , DOI: 10.1002/1873-3468.14018 Atsushi Kodan 1 , Ryota Futamata 2 , Yasuhisa Kimura 2 , Noriyuki Kioka 2 , Toru Nakatsu 3 , Hiroaki Kato 3 , Kazumitsu Ueda 1
FEBS Letters ( IF 3.5 ) Pub Date : 2020-12-11 , DOI: 10.1002/1873-3468.14018 Atsushi Kodan 1 , Ryota Futamata 2 , Yasuhisa Kimura 2 , Noriyuki Kioka 2 , Toru Nakatsu 3 , Hiroaki Kato 3 , Kazumitsu Ueda 1
Affiliation
|
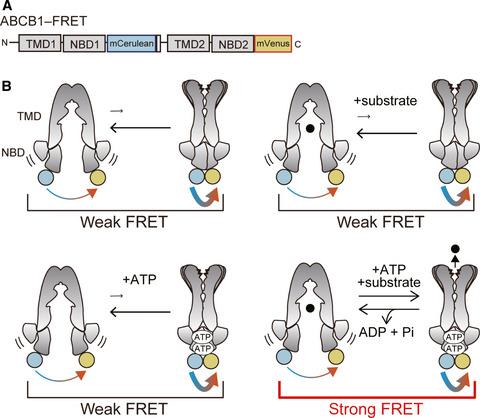
ABCB1, also called MDR1 or P-glycoprotein, exports various hydrophobic compounds and plays an essential role as a protective physiological barrier in several organs, including the brain, testis, and placenta. However, little is known about the structural mechanisms that allow ABCB1 to recognize hydrophobic compounds of diverse structures or the coupling of ATP hydrolysis to uphill substrate export. High-resolution X-ray crystal structures of the pre- and post-transport states and FRET analyses in living cells have revealed that an aromatic hydrophobic network at the top of the inner cavity is key for the conformational change of ABCB1 that is triggered by a hydrophobic substrate. ATP binding, but not hydrolysis, induces a progressive network that results in a twisting motion of the whole protein, squeezing out the substrate directly to the extracellular space. This twist-and-squeeze mechanism by which ABCB1 exports hydrophobic substrates is distinct from those of other transporters.
中文翻译:

ABCB1/MDR1/P-gp 采用依赖 ATP 的扭曲和挤压机制输出疏水性药物
ABCB1,也称为 MDR1 或 P-糖蛋白,输出各种疏水性化合物,并在多个器官(包括大脑、睾丸和胎盘)中作为保护性生理屏障发挥重要作用。然而,对于允许 ABCB1 识别不同结构的疏水化合物或 ATP 水解与上坡底物输出的耦合的结构机制知之甚少。活细胞中转运前和转运后状态的高分辨率 X 射线晶体结构和 FRET 分析表明,内腔顶部的芳香疏水网络是 ABCB1 构象变化的关键,由疏水底物。ATP 结合,而不是水解,诱导了一个渐进的网络,导致整个蛋白质的扭曲运动,将底物直接挤压到细胞外空间。ABCB1 输出疏水性底物的这种扭曲和挤压机制与其他转运蛋白的机制不同。
更新日期:2020-12-11
中文翻译:

ABCB1/MDR1/P-gp 采用依赖 ATP 的扭曲和挤压机制输出疏水性药物
ABCB1,也称为 MDR1 或 P-糖蛋白,输出各种疏水性化合物,并在多个器官(包括大脑、睾丸和胎盘)中作为保护性生理屏障发挥重要作用。然而,对于允许 ABCB1 识别不同结构的疏水化合物或 ATP 水解与上坡底物输出的耦合的结构机制知之甚少。活细胞中转运前和转运后状态的高分辨率 X 射线晶体结构和 FRET 分析表明,内腔顶部的芳香疏水网络是 ABCB1 构象变化的关键,由疏水底物。ATP 结合,而不是水解,诱导了一个渐进的网络,导致整个蛋白质的扭曲运动,将底物直接挤压到细胞外空间。ABCB1 输出疏水性底物的这种扭曲和挤压机制与其他转运蛋白的机制不同。



























 京公网安备 11010802027423号
京公网安备 11010802027423号