当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A structural model of the PriB–DnaT complex in Escherichia coli replication restart
FEBS Letters ( IF 3.0 ) Pub Date : 2020-12-13 , DOI: 10.1002/1873-3468.14020 Yoshito Abe 1, 2 , Yohei Ikeda 1 , Saki Fujiyama 1 , R Manjunatha Kini 3, 4 , Tadashi Ueda 1
FEBS Letters ( IF 3.0 ) Pub Date : 2020-12-13 , DOI: 10.1002/1873-3468.14020 Yoshito Abe 1, 2 , Yohei Ikeda 1 , Saki Fujiyama 1 , R Manjunatha Kini 3, 4 , Tadashi Ueda 1
Affiliation
|
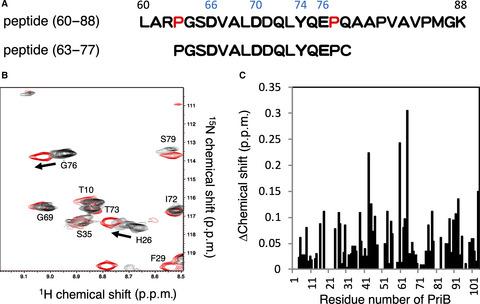
In Escherichia coli, DNA replication is restarted following DNA repair by the PriA-dependent pathway, in which the binding and dissociation of proteins such as PriA, PriB, and DnaT on ssDNA lead to the formation of a protein-DNA complex for recruiting the DnaB-DnaC replication protein complex. However, the structure of the PriB-DnaT complex, which is an essential step in the PriA-dependent pathway, remains elusive. In this study, the importance of His26 in PriB for replication restart was reconfirmed using plasmid complementation. Furthermore, we used NMR to examine the DnaT interaction sites on PriB. We also evaluated the PriB-DnaT peptide complex model, which was prepared by in silico docking, using molecular dynamics simulation. From these data, we propose a structural model that provides insight into the PriB-DnaT interaction.
中文翻译:

大肠杆菌复制重启中 PriB-DnaT 复合物的结构模型
在大肠杆菌中,DNA 复制在通过依赖 PriA 的途径进行 DNA 修复后重新开始,其中 PriA、PriB 和 DnaT 等蛋白质在 ssDNA 上的结合和解离导致形成用于募集 DnaB 的蛋白质-DNA 复合物-DnaC 复制蛋白复合物。然而,PriB-DnaT 复合物的结构是 PriA 依赖途径中的一个重要步骤,仍然难以捉摸。在这项研究中,使用质粒互补再次确认了 His26 在 PriB 中对复制重启的重要性。此外,我们使用 NMR 检查了 PriB 上的 DnaT 相互作用位点。我们还使用分子动力学模拟评估了通过计算机对接制备的 PriB-DnaT 肽复合模型。根据这些数据,我们提出了一个结构模型,可以深入了解 PriB-DnaT 相互作用。
更新日期:2020-12-13
中文翻译:

大肠杆菌复制重启中 PriB-DnaT 复合物的结构模型
在大肠杆菌中,DNA 复制在通过依赖 PriA 的途径进行 DNA 修复后重新开始,其中 PriA、PriB 和 DnaT 等蛋白质在 ssDNA 上的结合和解离导致形成用于募集 DnaB 的蛋白质-DNA 复合物-DnaC 复制蛋白复合物。然而,PriB-DnaT 复合物的结构是 PriA 依赖途径中的一个重要步骤,仍然难以捉摸。在这项研究中,使用质粒互补再次确认了 His26 在 PriB 中对复制重启的重要性。此外,我们使用 NMR 检查了 PriB 上的 DnaT 相互作用位点。我们还使用分子动力学模拟评估了通过计算机对接制备的 PriB-DnaT 肽复合模型。根据这些数据,我们提出了一个结构模型,可以深入了解 PriB-DnaT 相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号