当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electron injection for aromatic metamorphosis of indole
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-12-04 , DOI: 10.1002/jccs.202000369 Hideki Yorimitsu 1
Journal of the Chinese Chemical Society ( IF 1.6 ) Pub Date : 2020-12-04 , DOI: 10.1002/jccs.202000369 Hideki Yorimitsu 1
Affiliation
|
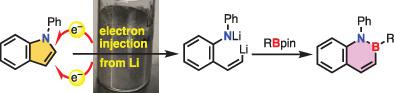
Aromatic metamorphosis, endocyclic transformations of aromatic compounds, has been emerging as a new synthetic strategy in organic synthesis. This counterintuitive strategy necessitates very powerful reactions that can surmount aromaticity and the strong carbon–heteroatom bonds of the heteroaromatic rings. This Short Account describes the development of the currently most powerful elementary reaction for aromatic metamorphosis, which is electron injection from lithium metal. Exceptionally robust and aromatic N‐phenylindole is subjected to the electron injection, resulting in the formation of the corresponding dianionic intermediate through reductive ring‐opening. A trapping reaction of the dianionic intermediate with organoboronic acid pinacol esters provides benzazaborines, which are attractive BN‐isosteres of naphthalenes. The electron injection helps establishing aromatic metamorphosis as a reliable synthetic methodology to provide novel useful molecules.
中文翻译:

电子注入用于吲哚的芳香变质
芳族化合物的芳香变态,环内转化已成为有机合成中一种新的合成策略。这种违反直觉的策略需要非常强大的反应,该反应可以超越芳香族原子和杂芳族环的强碳-杂原子键。该简短说明描述了当前最有效的芳香族变形反应的进展,该反应是从锂金属注入电子。异常坚固而芳香的N对苯基吲哚进行电子注入,通过还原性开环形成相应的双阴离子中间体。二价阴离子中间体与有机硼酸频哪醇酯的捕集反应提供了苯并氮杂萘啉,它们是萘的有吸引力的BN-等位异构体。电子注入有助于建立芳香族变态,作为提供新的有用分子的可靠合成方法。
更新日期:2020-12-04
中文翻译:

电子注入用于吲哚的芳香变质
芳族化合物的芳香变态,环内转化已成为有机合成中一种新的合成策略。这种违反直觉的策略需要非常强大的反应,该反应可以超越芳香族原子和杂芳族环的强碳-杂原子键。该简短说明描述了当前最有效的芳香族变形反应的进展,该反应是从锂金属注入电子。异常坚固而芳香的N对苯基吲哚进行电子注入,通过还原性开环形成相应的双阴离子中间体。二价阴离子中间体与有机硼酸频哪醇酯的捕集反应提供了苯并氮杂萘啉,它们是萘的有吸引力的BN-等位异构体。电子注入有助于建立芳香族变态,作为提供新的有用分子的可靠合成方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号