当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Getting a Grip on the Undrugged: Targeting β‐Catenin with Fragment‐Based Methods
ChemMedChem ( IF 3.6 ) Pub Date : 2020-12-04 , DOI: 10.1002/cmdc.202000839 Dirk Kessler 1 , Moriz Mayer 1 , Stephan K Zahn 1 , Markus Zeeb 2 , Simon Wöhrle 1 , Andreas Bergner 1 , Jens Bruchhaus 1 , Tuncay Ciftci 2 , Georg Dahmann 2 , Maike Dettling 1 , Sandra Döbel 1 , Julian E Fuchs 1 , Leonhard Geist 1 , Wolfgang Hela 1 , Christiane Kofink 1 , Roland Kousek 1 , Franziska Moser 2 , Teresa Puchner 1 , Klaus Rumpel 1 , Maximilian Scharnweber 1 , Patrick Werni 1 , Bernhard Wolkerstorfer 1 , Dennis Breitsprecher 3, 4 , Philipp Baaske 3 , Mark Pearson 1 , Darryl B McConnell 1 , Jark Böttcher 1
ChemMedChem ( IF 3.6 ) Pub Date : 2020-12-04 , DOI: 10.1002/cmdc.202000839 Dirk Kessler 1 , Moriz Mayer 1 , Stephan K Zahn 1 , Markus Zeeb 2 , Simon Wöhrle 1 , Andreas Bergner 1 , Jens Bruchhaus 1 , Tuncay Ciftci 2 , Georg Dahmann 2 , Maike Dettling 1 , Sandra Döbel 1 , Julian E Fuchs 1 , Leonhard Geist 1 , Wolfgang Hela 1 , Christiane Kofink 1 , Roland Kousek 1 , Franziska Moser 2 , Teresa Puchner 1 , Klaus Rumpel 1 , Maximilian Scharnweber 1 , Patrick Werni 1 , Bernhard Wolkerstorfer 1 , Dennis Breitsprecher 3, 4 , Philipp Baaske 3 , Mark Pearson 1 , Darryl B McConnell 1 , Jark Böttcher 1
Affiliation
|
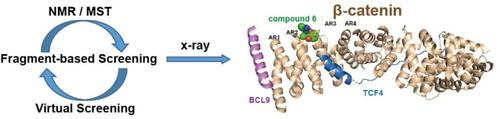
Aberrant WNT pathway activation, leading to nuclear accumulation of β‐catenin, is a key oncogenic driver event. Mutations in the tumor suppressor gene APC lead to impaired proteasomal degradation of β‐catenin and subsequent nuclear translocation. Restoring cellular degradation of β‐catenin represents a potential therapeutic strategy. Here, we report the fragment‐based discovery of a small molecule binder to β‐catenin, including the structural elucidation of the binding mode by X‐ray crystallography. The difficulty in drugging β‐catenin was confirmed as the primary screening campaigns identified only few and very weak hits. Iterative virtual and NMR screening techniques were required to discover a compound with sufficient potency to be able to obtain an X‐ray co‐crystal structure. The binding site is located between armadillo repeats two and three, adjacent to the BCL9 and TCF4 binding sites. Genetic studies show that it is unlikely to be useful for the development of protein–protein interaction inhibitors but structural information and established assays provide a solid basis for a prospective optimization towards β‐catenin proteolysis targeting chimeras (PROTACs) as alternative modality.
中文翻译:

掌控非药物:使用基于片段的方法靶向 β-连环蛋白
异常的 WNT 通路激活导致 β-连环蛋白的核积累,是一个关键的致癌驱动事件。肿瘤抑制基因 APC 的突变导致 β-连环蛋白的蛋白酶体降解受损以及随后的核转位。恢复β-连环蛋白的细胞降解是一种潜在的治疗策略。在这里,我们报告了基于片段的 β-连环蛋白小分子结合剂的发现,包括通过 X 射线晶体学对结合模式的结构阐明。由于初步筛选活动仅发现了很少且非常微弱的命中,因此证实了对 β-连环蛋白进行药物治疗的难度。需要迭代虚拟和核磁共振筛选技术来发现具有足够效力的化合物,从而能够获得 X 射线共晶结构。结合位点位于犰狳重复序列二和三之间,邻近 BCL9 和 TCF4 结合位点。遗传学研究表明,它不太可能对蛋白质-蛋白质相互作用抑制剂的开发有用,但结构信息和已建立的检测方法为β-连环蛋白蛋白水解靶向嵌合体(PROTAC)作为替代方式的前瞻性优化提供了坚实的基础。
更新日期:2020-12-04
中文翻译:

掌控非药物:使用基于片段的方法靶向 β-连环蛋白
异常的 WNT 通路激活导致 β-连环蛋白的核积累,是一个关键的致癌驱动事件。肿瘤抑制基因 APC 的突变导致 β-连环蛋白的蛋白酶体降解受损以及随后的核转位。恢复β-连环蛋白的细胞降解是一种潜在的治疗策略。在这里,我们报告了基于片段的 β-连环蛋白小分子结合剂的发现,包括通过 X 射线晶体学对结合模式的结构阐明。由于初步筛选活动仅发现了很少且非常微弱的命中,因此证实了对 β-连环蛋白进行药物治疗的难度。需要迭代虚拟和核磁共振筛选技术来发现具有足够效力的化合物,从而能够获得 X 射线共晶结构。结合位点位于犰狳重复序列二和三之间,邻近 BCL9 和 TCF4 结合位点。遗传学研究表明,它不太可能对蛋白质-蛋白质相互作用抑制剂的开发有用,但结构信息和已建立的检测方法为β-连环蛋白蛋白水解靶向嵌合体(PROTAC)作为替代方式的前瞻性优化提供了坚实的基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号