当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, molecular docking and preliminary antileukemic activity of 4‐methoxybenzyl derivatives bearing an imidazo[2,1‐b][1,3,4]thiadiazoles
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2021-01-07 , DOI: 10.1002/cbdv.202000800 B Choodamani 1, 2 , Karla G Cano Hernandez 3 , Sujeet Kumar 1 , Ann Maria Tony 4 , Austre Y Schiaffino Bustamante 3 , Renato J Aguilera 3 , Dominique Schols 5 , C Gopi Mohan 4 , Subhas S Karki 1, 2
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2021-01-07 , DOI: 10.1002/cbdv.202000800 B Choodamani 1, 2 , Karla G Cano Hernandez 3 , Sujeet Kumar 1 , Ann Maria Tony 4 , Austre Y Schiaffino Bustamante 3 , Renato J Aguilera 3 , Dominique Schols 5 , C Gopi Mohan 4 , Subhas S Karki 1, 2
Affiliation
|
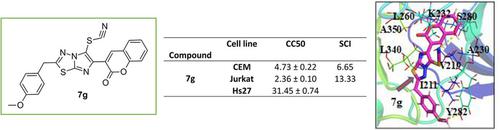
In this study, we synthesized 22 compounds in a series with various substitution on imidazo[2,1- b ][1,3,4]thiadiazoles. The potential cytotoxic activity of these compounds investigated in leukemia cell lines by Differential Nuclear Staining (DNS). Our results identify two compounds 2-(4-methoxybenzyl)-6-(2-oxo-2H-chromen-3-yl)imidazo[2,1-b][1,3,4]thiadiazol-5-yl thiocyanate and 6-(4-chlorophenyl)-2-(4'-methoxybenzyl)imidazo[2,1-b][1,3,4]thiadiazole-5-carbaldehyde exhibited as the most cytotoxic effect against murine leukemia cells (L1210), human T-lymphocyte cells (CEM) and human cervix carcinoma cells (HeLa) with IC 50 values ranging between 0.79 to 1.6 μM. The results indicate that compound 2-(4-methoxybenzyl)-6-(2-oxo-2H-chromen-3-yl)imidazo[2,1-b][1,3,4]thiadiazol-5-yl thiocyanate is inducing phosphatidylserine externalization and caspase-3 activation which are both a hallmark of apoptosis. Docking studies showed that compound 2-(4-methoxybenzyl)-6-(2-oxo-2H-chromen-3-yl)imidazo[2,1-b][1,3,4]thiadiazol-5-yl thiocyanate binds within the active sites of transforming growth factor beta (TGF- β) type I receptor kinase domain by strong hydrogen binding and hydrophobic interactions.
中文翻译:

咪唑并[2,1-b][1,3,4]噻二唑类4-甲氧基苯甲基衍生物的合成、分子对接及初步抗白血病活性
在本研究中,我们合成了 22 种在咪唑并[2,1-b][1,3,4]噻二唑上进行不同取代的系列化合物。通过差异核染色 (DNS) 研究了这些化合物在白血病细胞系中的潜在细胞毒活性。我们的结果鉴定出两种化合物 2-(4-methoxybenzyl)-6-(2-oxo-2H-chromen-3-yl)imidazo[2,1-b][1,3,4]thiadiazol-5-yl thiocyanate 和6-(4-氯苯基)-2-(4'-甲氧基苯甲基)咪唑并[2,1-b][1,3,4]噻二唑-5-甲醛对小鼠白血病细胞(L1210)表现出最强的细胞毒性作用,人 T 淋巴细胞 (CEM) 和人宫颈癌细胞 (HeLa),IC 50 值范围在 0.79 至 1.6 μM 之间。结果表明,化合物2-(4-甲氧基苄基)-6-(2-氧代-2H-苯并吡喃-3-基)咪唑并[2,1-b][1,3,4]噻二唑-5-基硫氰酸酯为诱导磷脂酰丝氨酸外化和 caspase-3 激活,这两者都是细胞凋亡的标志。对接研究表明,化合物 2-(4-methoxybenzyl)-6-(2-oxo-2H-chromen-3-yl)imidazo[2,1-b][1,3,4]thiadiazol-5-yl thiocyanate 结合通过强氢结合和疏水相互作用,位于转化生长因子β (TGF-β) I 型受体激酶结构域的活性位点内。
更新日期:2021-01-07
中文翻译:

咪唑并[2,1-b][1,3,4]噻二唑类4-甲氧基苯甲基衍生物的合成、分子对接及初步抗白血病活性
在本研究中,我们合成了 22 种在咪唑并[2,1-b][1,3,4]噻二唑上进行不同取代的系列化合物。通过差异核染色 (DNS) 研究了这些化合物在白血病细胞系中的潜在细胞毒活性。我们的结果鉴定出两种化合物 2-(4-methoxybenzyl)-6-(2-oxo-2H-chromen-3-yl)imidazo[2,1-b][1,3,4]thiadiazol-5-yl thiocyanate 和6-(4-氯苯基)-2-(4'-甲氧基苯甲基)咪唑并[2,1-b][1,3,4]噻二唑-5-甲醛对小鼠白血病细胞(L1210)表现出最强的细胞毒性作用,人 T 淋巴细胞 (CEM) 和人宫颈癌细胞 (HeLa),IC 50 值范围在 0.79 至 1.6 μM 之间。结果表明,化合物2-(4-甲氧基苄基)-6-(2-氧代-2H-苯并吡喃-3-基)咪唑并[2,1-b][1,3,4]噻二唑-5-基硫氰酸酯为诱导磷脂酰丝氨酸外化和 caspase-3 激活,这两者都是细胞凋亡的标志。对接研究表明,化合物 2-(4-methoxybenzyl)-6-(2-oxo-2H-chromen-3-yl)imidazo[2,1-b][1,3,4]thiadiazol-5-yl thiocyanate 结合通过强氢结合和疏水相互作用,位于转化生长因子β (TGF-β) I 型受体激酶结构域的活性位点内。











































 京公网安备 11010802027423号
京公网安备 11010802027423号