当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diastereodivergent Synthesis of β‐Amino Alcohols by Dual‐Metal‐Catalyzed Coupling of Alkoxyallenes with Aldimine Esters
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-12-03 , DOI: 10.1002/anie.202014510 Weiwei Zi 1 , Minghui Zhu 2 , Qinglong Zhang 2
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-12-03 , DOI: 10.1002/anie.202014510 Weiwei Zi 1 , Minghui Zhu 2 , Qinglong Zhang 2
Affiliation
|
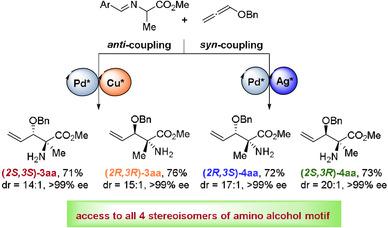
Both syn‐ and anti‐β‐amino alcohols are common structural motifs in natural products, drug molecules, chiral ligands and catalysts. However, the currently available methods for synthesizing these motifs are limited to generate only one diastereoisomer. Therefore, development of a unified method for stereoselective access to complementary diastereomers would be highly desirable. Herein, we report a method for dual‐metal‐catalyzed diastereodivergent coupling of alkoxyallenes with aldimine esters. By carefully selecting the two metals and appropriate chiral ligands, we could synthesize both syn‐ and anti‐β‐amino alcohol motifs with high enantioselectivity and diastereoselectivity from the same set of starting materials. Furthermore, stereodivergent syntheses of all four stereoisomers of β‐amino alcohols could be achieved. We demonstrated the synthetic utility of this method by concisely synthesizing two β‐amino alcohol natural products, mycestericins F and G.
中文翻译:

双金属催化的烷氧基烯与醛亚胺酯的偶合反应合成β-氨基醇的非对映异构体
两个顺式-和反-β -氨基醇是在天然产物,药物分子,手性配体和催化剂共同的结构基序。但是,合成这些基序的当前可用方法仅限于仅产生一种非对映异构体。因此,非常需要开发一种立体方法,以选择性地互补互补非对映异构体。本文中,我们报告了烷氧基丙二烯与醛亚胺酯的双金属催化非对映异构偶联方法。通过仔细选择两种金属和合适的手性配体,我们可以合成顺式和反式来自同一套起始材料的具有高对映选择性和非对映选择性的β-氨基醇基序。此外,可以实现β-氨基醇的所有四种立体异构体的立体发散性合成。我们通过简洁地合成两种β-氨基醇天然产物Mycestericins F和G证明了该方法的合成效用。
更新日期:2020-12-03
中文翻译:

双金属催化的烷氧基烯与醛亚胺酯的偶合反应合成β-氨基醇的非对映异构体
两个顺式-和反-β -氨基醇是在天然产物,药物分子,手性配体和催化剂共同的结构基序。但是,合成这些基序的当前可用方法仅限于仅产生一种非对映异构体。因此,非常需要开发一种立体方法,以选择性地互补互补非对映异构体。本文中,我们报告了烷氧基丙二烯与醛亚胺酯的双金属催化非对映异构偶联方法。通过仔细选择两种金属和合适的手性配体,我们可以合成顺式和反式来自同一套起始材料的具有高对映选择性和非对映选择性的β-氨基醇基序。此外,可以实现β-氨基醇的所有四种立体异构体的立体发散性合成。我们通过简洁地合成两种β-氨基醇天然产物Mycestericins F和G证明了该方法的合成效用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号