Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2020-12-04 , DOI: 10.1016/j.jhazmat.2020.124766 Weirui Chen , Jinxin Xie , Xukai Li , Laisheng Li
|
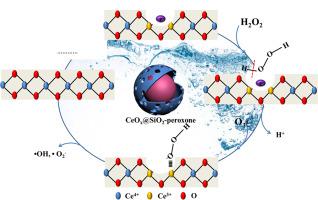
The low efficiency of peroxone (O3/H2O2) at acidic and neutral pH restrained its application in water purification. To overcome this shortcoming, CeOX@SiO2 with large surface area, abundant surface oxygen vacancies (Vo), Lewis sites (L sites) and high Ce(III)/Ce(IV) ratio were synthesized to change the traditional electron transfer pathway between O3 and H2O2. Vo was facile in absorbing H2O2 to form Vo-H2O2 and L sites were capable of absorbing O3 to form L-O3. The electron at Vo could be donated to Vo-H2O2 and generate Vo-HO2−, which then effectively triggered the decomposition of L-O3 at CeOX@SiO2’s interface and O3 in bulk solution. The electron transfer at the solid-liquid interface with the help of Ce3+/Ce4+ redox cycle and Vo was pH independent and different from the traditional electron transfer of peroxone reaction. Nitrobenzene (NB) mineralization was promoted to 92.5% in CeOX@SiO2-peroxone, but only 63.8% TOC was removed in tradition peroxone process. Moreover, CeOX@SiO2-peroxone had a wide pH application range. NB’s degradation in CeOX@SiO2-peroxone process followed the co-oxidation mechanism of superoxide free (•O2−) and hydroxyl radical (•OH). The finding of this study could broaden the popularization of peroxone in water treatment and provided a strategy for catalyst design.
中文翻译:

氧空位和路易斯位点通过在CeO x @SiO 2上的表面电子转移而在宽pH范围内激活O 3 / H 2 O 2,从而使硝基苯矿化
在酸性和中性pH值下,Peroxone(O 3 / H 2 O 2)的效率低,限制了其在水净化中的应用。为了克服这一缺点,合成了具有大表面积,丰富的表面氧空位(V o),路易斯位点(L位)和高的Ce(III)/ Ce(IV)比的CeO X @SiO 2来改变传统的电子转移O 3和H 2 O 2之间的通道。V o易于吸收H 2 O 2形成V o -H 2 O 2,并且L位能够吸收O3形成LO 3。在V电子ø可以捐赠到V ö -H 2 ö 2和生成V ö -HO 2 - ,其然后引发有效LO的分解3在的CeO X @SiO 2的接口和O 3在本体溶液中。在Ce 3+ / Ce 4+氧化还原循环和V o的帮助下,固液界面的电子转移不受pH值的影响,与传统的过氧化物反应的电子转移不同。CeO中的硝基苯(NB)矿化作用提高至92.5%X @SiO 2 -peroxone,但在传统的peroxone工艺中仅去除了63.8%的TOC。此外,CeO X @SiO 2-过氧化物具有广泛的pH应用范围。NB的以CeO降解X @SiO 2 -peroxone过程随后超氧自由的共氧化机制(•Ø 2 - )和羟基自由基(OH•)。这项研究的发现可以扩大过氧乐酮在水处理中的普及,并为催化剂设计提供了一种策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号