Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-12-03 , DOI: 10.1016/j.jcat.2020.11.035 Niels D. Nielsen , Anker D. Jensen , Jakob M. Christensen
|
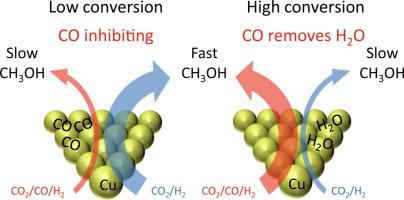
The roles of CO and CO2 in Cu-catalyzed methanol synthesis from syngas were evaluated in experiments with gas switching between CO/H2 and CO2/H2 feeds and between a CO2/CO/H2 feed and a corresponding CO-free CO2/inert/H2 feed. Switching between CO/Ar/H2 (3/29/68) and CO2/N2/H2 (3/29/68) for Cu/Al2O3 showed that the rate of methanol synthesis on Cu is more than an order of magnitude higher from CO2 compared to CO. Experiments switching between CO2/CO/H2 and CO2/inert/H2 showed that at low conversion conditions with negligible product formation, CO is purely inhibiting for Raney Cu and for a range of supported (on SiO2, TiO2, Al2O3 and ZnO) Cu-catalysts including an industrial-type Cu/ZnO/Al2O3 catalyst. Given the generality across Cu-based samples the mechanism of this inhibition is most likely competitive adsorption of CO on the Cu surface. However, as conversion is increased by lowering the gas space velocity there is a sharp transition from an inhibiting to a beneficial role of CO relative to a CO-free feed. With increasing conversion more water is formed, and as water is a far stronger inhibitor to Cu-based catalysts than CO, the beneficial effect of CO arises from the removal of water through the water–gas shift reaction. At low conversion the methanol synthesis rate is thus highest for a CO-free feed that minimizes CO inhibition, whereas the rate at high conversion is optimal with a CO-rich syngas that minimizes water inhibition. The transition between these two types of behavior occurs around the conversion range leading to 1 mol% of methanol in the effluent gas. Hence, CO has a beneficial role at commercial high conversion conditions. The ZnO support exerts a strong, beneficial support effect at low conversion conditions, where the strong reductant CO has a purely negative effect. This could suggest that reduced Zn-sites (oxygen vacancies in ZnO or Cu-Zn surface alloy sites), whose concentration are expected to depend on the reductive potential of the atmosphere, are not critical to the support effect from ZnO. At both industrial conditions (523 K, 50 bar), mild conditions (448 K, atm. pressure) and in a nominally oxidizing gas (498 K, 20 bar with CO2 > H2) the addition of CO to the feed is detrimental to the activity of Cu/ZnO/Al2O3 at low conversion conditions. This supports that CO plays no beneficial role by facilitating ZnO-reduction and possibly that Zn alloyed into the Cu surface is unimportant for catalytic activity.
中文翻译:

CO和CO 2在铜基催化剂上高压甲醇合成中的作用
通过在CO / H 2和CO 2 / H 2进料之间以及CO 2 / CO / H 2进料和相应的CO-之间进行气体切换的实验中,评估了CO和CO 2在合成气中Cu催化的甲醇合成中的作用。免费的CO 2 /惰性/ H 2进料。Cu / Al 2 O 3在CO / Ar / H 2(3/29/68)和CO 2 / N 2 / H 2(3/29/68)之间切换表明,甲醇在Cu上的合成速率大于与CO相比,CO 2的数量级更高。实验在CO之间进行切换2 / CO / H 2和CO 2 /惰性/ H 2表明,在低转化率条件下且产物形成可忽略不计,CO完全抑制了阮内铜和一定范围的负载(SiO 2,TiO 2,Al 2 O 3)和ZnO)Cu催化剂,包括工业类型的Cu / ZnO / Al 2 O 3催化剂。考虑到跨铜基样品的普遍性,这种抑制的机制很可能是CO在铜表面上的竞争性吸附。但是,由于通过降低气体空间速度来提高转化率,相对于无CO进料,CO的抑制作用从陡峭的转变为有益的作用。随着转化率的增加,会形成更多的水,并且由于水是对铜基催化剂的强于一氧化碳的抑制剂,因此,通过水煤气变换反应除去水,会产生一氧化碳的有益作用。因此,在低转化率下,无CO进料的甲醇合成速率最高,这会使CO抑制作用最小化,而在高转化率下,富含CO的合成气可使水抑制作用最小化是最佳的。这两种行为之间的过渡发生在转化率范围内,导致流出气体中的甲醇含量为1 mol%。因此,CO在商业高转化率条件下具有有益的作用。ZnO载体在低转化条件下发挥强大的有益载体作用,其中强还原剂CO纯粹具有负面作用。这可能表明减少的锌位点(ZnO或Cu-Zn表面合金位点中的氧空位)的浓度预计取决于大气的还原电位,对于ZnO的支持作用并不是至关重要的。在两种工业条件(523 K,50 bar),中等条件(448 K,大气压)和名义氧化性气体(498 K,20 bar,含CO)下 ZnO载体在低转化条件下发挥强大的有益载体作用,其中强还原剂CO纯粹具有负面作用。这可能表明减少的锌位点(ZnO或Cu-Zn表面合金位点中的氧空位)的浓度预计取决于大气的还原电位,对于ZnO的支持作用并不是至关重要的。在两种工业条件(523 K,50 bar),中等条件(448 K,大气压)和名义氧化性气体(498 K,20 bar,含CO)下 ZnO载体在低转化条件下发挥强大的有益载体作用,其中强还原剂CO纯粹具有负面作用。这可能表明减少的锌位点(ZnO或Cu-Zn表面合金位点中的氧空位)的浓度预计取决于大气的还原电位,对于ZnO的支持作用并不是至关重要的。在两种工业条件(523 K,50 bar),中等条件(448 K,大气压)和名义氧化性气体(498 K,20 bar,含CO)下 对于ZnO的支持效果而言,并不是至关重要的。在两种工业条件(523 K,50 bar),中等条件(448 K,大气压)和名义氧化性气体(498 K,20 bar,含CO)下 对于ZnO的支持效果而言,并不是至关重要的。在两种工业条件(523 K,50 bar),中等条件(448 K,大气压)和名义氧化性气体(498 K,20 bar,含CO)下2 > H 2)在进料中添加CO不利于低转化率条件下Cu / ZnO / Al 2 O 3的活性。这表明CO不会通过促进ZnO还原而发挥有益作用,并且可能合金化到Cu表面的Zn对于催化活性并不重要。











































 京公网安备 11010802027423号
京公网安备 11010802027423号