Journal of Catalysis ( IF 6.5 ) Pub Date : 2020-12-03 , DOI: 10.1016/j.jcat.2020.11.026 Sean R. Noble , Sean E. Barnes , Ritubarna Banerjee , Jeff Miller , John R. Regalbuto
|
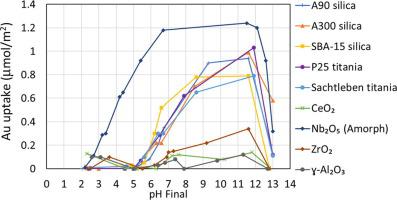
Gold bis-ethylenediamine ([Au(en)2]3+ or AuED) is an effective precursor for the preparation of small Au nanoparticles on common catalyst supports. By studying the adsorption of AuED over a wide variety of supports with surveys of uptake versus time and pH, including XAS studies of solution and adsorbed species, we have demonstrated that an electrostatic mechanism accounts for the data much better than the previously postulated cation exchange mechanism. In the electrostatic mechanism 1) uptake-pH curves are volcano-shaped and are higher in amplitude and broader in pH the lower the PZC of the support, 2) AuED complexes retain at least one hydration sheath with diameter 13.3 Å (0.72 complex/nm2) and adsorb as outer sphere complexes, 3) the monolayer limit in aqueous solutions basified with NaOH is not determined by cation exchange stoichiometry but is a steric limit of close-packed, hydrated complexes. They adsorb at a much lower surface density than that of the surface hydroxyl groups and never electrically saturate the surface. 4) The change in AuED speciation at high pH is consistent with a change in the coulombic attraction of a 3+ versus a 2+ complex.
Over carbon supports, adsorption is predominantly electrostatic, but an additional parallel mechanism is apparent in proportion to the graphitic nature of the support.
Synthesized Au nanoparticles over the oxide and carbon materials was characterized by high sensitivity powder XRD, STEM imaging, and TPR. The resultant nanoparticles were generally smaller than other synthesis methods reported in the literature for silica, titania, alumina, and carbon. The loading limit of electrostatic adsorption is a surface density of about 1 μmol/m2. At loadings less than or equal to this, electrostatic adsorption provides a simple method with which to synthesize well dispersed Au nanoparticles.
中文翻译:

金双乙二胺支持的纳米粒子合成:吸附到氧化物和碳上的机制
金双乙二胺([Au(en)2 ] 3+或AuED)是在常规催化剂载体上制备小Au纳米颗粒的有效前体。通过研究摄取量随时间和pH的变化,包括在溶液和吸附物质的XAS研究中,研究了AuED在各种载体上的吸附情况,我们证明了静电机理比以前假设的阳离子交换机理要好得多。 。在静电机理中:1)吸收pH曲线呈火山状,振幅较高,pH越宽,载体的PZC越低; 2)AuED络合物保留至少一个直径为13.3Å(0.72 complex / nm)的水合鞘2)并吸附为外球络合物,3)用NaOH碱化的水溶液中的单层极限不是由阳离子交换化学计量确定的,而是密堆积的水合配合物的空间极限。它们以比表面羟基低得多的表面密度吸附,并且从未使表面电饱和。4)在高pH下AuED形态的变化与3+与2+复合物的库仑吸引率变化一致。
在碳载体上,吸附主要是静电,但与载体的石墨性质成比例,显然还有其他平行机制。
通过高灵敏度粉末XRD,STEM成像和TPR表征了在氧化物和碳材料上合成的Au纳米颗粒。所得的纳米颗粒通常小于文献中报道的其他二氧化硅,二氧化钛,氧化铝和碳的合成方法。静电吸附的负载极限是约1μmol/ m 2的表面密度。在小于或等于此的负载量下,静电吸附提供了一种合成分散良好的Au纳米颗粒的简单方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号