Cell Metabolism ( IF 27.7 ) Pub Date : 2020-12-04 , DOI: 10.1016/j.cmet.2020.11.008 Jonathan R Brestoff 1 , Craig B Wilen 2 , John R Moley 1 , Yongjia Li 1 , Wei Zou 1 , Nicole P Malvin 1 , Marina N Rowen 1 , Brian T Saunders 1 , Hongming Ma 3 , Madison R Mack 4 , Barry L Hykes 1 , Dale R Balce 5 , Anthony Orvedahl 6 , Jesse W Williams 1 , Nidhi Rohatgi 1 , Xiaoyan Wang 1 , Michael R McAllaster 1 , Scott A Handley 1 , Brian S Kim 7 , John G Doench 8 , Bernd H Zinselmeyer 1 , Michael S Diamond 9 , Herbert W Virgin 10 , Andrew E Gelman 11 , Steven L Teitelbaum 12
|
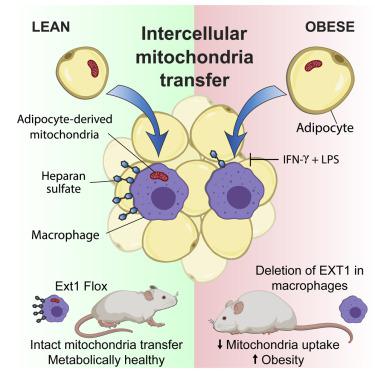
Recent studies suggest that mitochondria can be transferred between cells to support the survival of metabolically compromised cells. However, whether intercellular mitochondria transfer occurs in white adipose tissue (WAT) or regulates metabolic homeostasis in vivo remains unknown. We found that macrophages acquire mitochondria from neighboring adipocytes in vivo and that this process defines a transcriptionally distinct macrophage subpopulation. A genome-wide CRISPR-Cas9 knockout screen revealed that mitochondria uptake depends on heparan sulfates (HS). High-fat diet (HFD)-induced obese mice exhibit lower HS levels on WAT macrophages and decreased intercellular mitochondria transfer from adipocytes to macrophages. Deletion of the HS biosynthetic gene Ext1 in myeloid cells decreases mitochondria uptake by WAT macrophages, increases WAT mass, lowers energy expenditure, and exacerbates HFD-induced obesity in vivo. Collectively, this study suggests that adipocytes and macrophages employ intercellular mitochondria transfer as a mechanism of immunometabolic crosstalk that regulates metabolic homeostasis and is impaired in obesity.
中文翻译:

细胞间线粒体转移至巨噬细胞调节白色脂肪组织稳态并在肥胖中受损
最近的研究表明,线粒体可以在细胞之间转移,以支持代谢受损的细胞的生存。然而,细胞间线粒体转移是否发生在白色脂肪组织(WAT)或调节体内代谢稳态仍不清楚。我们发现巨噬细胞从体内邻近的脂肪细胞获取线粒体,并且这个过程定义了转录上不同的巨噬细胞亚群。全基因组 CRISPR-Cas9 敲除筛选显示线粒体的摄取取决于硫酸乙酰肝素 (HS)。高脂饮食 (HFD) 诱导的肥胖小鼠在 WAT 巨噬细胞上表现出较低的 HS 水平,并且从脂肪细胞到巨噬细胞的细胞间线粒体转移减少。骨髓细胞中 HS 生物合成基因Ext1的缺失会减少 WAT 巨噬细胞对线粒体的摄取,增加 WAT 质量,降低能量消耗,并加剧 HFD 诱导的体内肥胖。总的来说,这项研究表明脂肪细胞和巨噬细胞利用细胞间线粒体转移作为免疫代谢串扰的机制,调节代谢稳态并在肥胖中受损。











































 京公网安备 11010802027423号
京公网安备 11010802027423号