当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dehydrogenation and Rehydrogenation of Ammonia Borane under Shock Loading: Ab Initio Molecular Dynamics Simulations
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-12-03 , DOI: 10.1021/acs.jpcc.0c07748 Yao-Yao Huang 1 , Lin-Xiang Ji 2 , Lan-Ting Shi 1 , Hai-Chao Ren 1 , Zheng-Hua He 1 , Guang-Fu Ji 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2020-12-03 , DOI: 10.1021/acs.jpcc.0c07748 Yao-Yao Huang 1 , Lin-Xiang Ji 2 , Lan-Ting Shi 1 , Hai-Chao Ren 1 , Zheng-Hua He 1 , Guang-Fu Ji 1
Affiliation
|
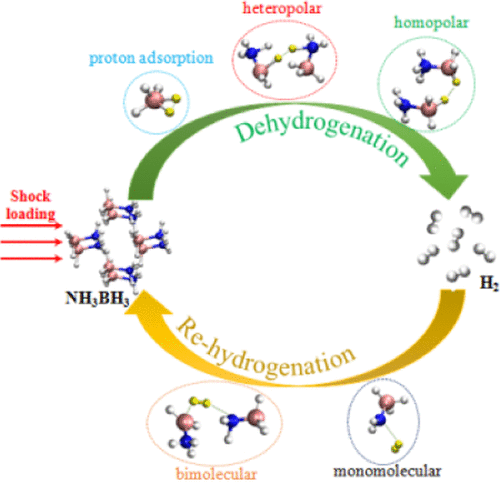
Ammonia borane (AB, NH3BH3), as a hydrogen-rich material, has been attracting great interest in the field of hydrogen storage. However, the mechanism of its dehydrogenation and rehydrogenation is not sufficiently understood yet. In this work, the initial decomposition process of AB under shock loading is investigated using the ab initio molecular dynamics method. The results show that the B–H bond breaking plays a more important role in the reaction initiation. Three main reaction pathways for H2 release are revealed. (I) Heteropolar dihydrogen interaction (N-Hδ+···δ−H–B) and (II) homopolar dihydrogen interaction (B–Hδ−···δ−H–B) are still the most popular reaction mechanisms. However, a direct hydrogen adsorption and H2 liberation mechanism (III) is uncovered. The H radical readily adsorbs a B atom to form the pentacoordinate boron-containing species and efficiently activates the adjacent B–H bond, which further ruptures to form H2 molecules. What is more, we discover the unexpected hydrogenation behaviors between new-formed H2 and BNH compounds. In addition, the similar H exchange reactions assisted by H2 are also explored. The probable rehydrogenation mechanisms are proposed based on our results. These theoretical calculations not only give a comprehensive understanding about AB decomposition under shock loading but also shed light on its rehydrogenation for hydrogen storage.
中文翻译:

冲击载荷下氨硼烷的脱氢和再氢化:从头算分子动力学模拟
作为富氢材料的氨硼烷(AB,NH 3 BH 3)在储氢领域引起了极大的兴趣。但是,对其脱氢和再氢化的机理还没有足够的了解。在这项工作中,使用从头算分子动力学方法研究了AB在冲击载荷下的初始分解过程。结果表明,B–H键断裂在反应引发中起着更重要的作用。揭示了H 2释放的三个主要反应途径。(I)极性基二氢相互作用(NH δ+ ··· δ- H-B)和(II)单极二氢相互作用(B-H δ- ··· δ-HB)仍然是最流行的反应机制。然而,没有发现直接的氢吸附和H 2释放机理(III)。H自由基很容易吸附B原子以形成五配位含硼物质,并有效地激活相邻的BH键,从而进一步断裂形成H 2分子。此外,我们发现了新型H 2和BNH化合物之间的意外氢化行为。此外,由H 2辅助的类似的H交换反应也进行了探索。根据我们的研究结果,提出了可能的加氢机理。这些理论计算不仅对冲击载荷下的AB分解有一个全面的了解,而且为AB的氢再生提供了启示。
更新日期:2020-12-17
中文翻译:

冲击载荷下氨硼烷的脱氢和再氢化:从头算分子动力学模拟
作为富氢材料的氨硼烷(AB,NH 3 BH 3)在储氢领域引起了极大的兴趣。但是,对其脱氢和再氢化的机理还没有足够的了解。在这项工作中,使用从头算分子动力学方法研究了AB在冲击载荷下的初始分解过程。结果表明,B–H键断裂在反应引发中起着更重要的作用。揭示了H 2释放的三个主要反应途径。(I)极性基二氢相互作用(NH δ+ ··· δ- H-B)和(II)单极二氢相互作用(B-H δ- ··· δ-HB)仍然是最流行的反应机制。然而,没有发现直接的氢吸附和H 2释放机理(III)。H自由基很容易吸附B原子以形成五配位含硼物质,并有效地激活相邻的BH键,从而进一步断裂形成H 2分子。此外,我们发现了新型H 2和BNH化合物之间的意外氢化行为。此外,由H 2辅助的类似的H交换反应也进行了探索。根据我们的研究结果,提出了可能的加氢机理。这些理论计算不仅对冲击载荷下的AB分解有一个全面的了解,而且为AB的氢再生提供了启示。











































 京公网安备 11010802027423号
京公网安备 11010802027423号