当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nucleosome Dynamics during Transcription Elongation
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-12-02 , DOI: 10.1021/acschembio.0c00617 Mai T. Huynh , Satya P. Yadav , Joseph C. Reese , Tae-Hee Lee
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-12-02 , DOI: 10.1021/acschembio.0c00617 Mai T. Huynh , Satya P. Yadav , Joseph C. Reese , Tae-Hee Lee
|
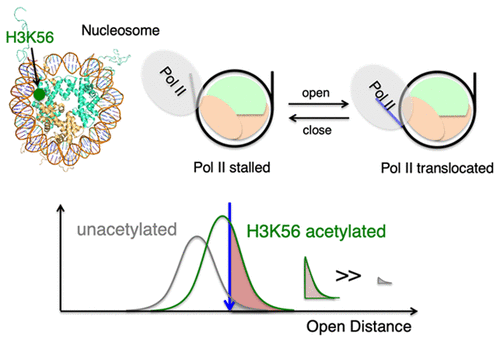
The nucleosome is the basic packing unit of the eukaryotic genome. Dynamic interactions between DNA and histones in the nucleosome are the molecular basis of gene accessibility regulation that governs the kinetics of various DNA-templated processes such as transcription elongation by RNA Polymerase II (Pol II). On the basis of single-molecule FRET measurements with chemically modified histones, we investigated the nucleosome dynamics during transcription elongation and how it is affected by histone acetylation at H3 K56 and the histone chaperone Nap1, both of which can affect DNA–histone interactions. We observed that H3K56 acetylation dramatically shortens the pause duration of Pol II near the entry region of the nucleosome, while Nap1 induces no noticeable difference. We also found that the elongation rate of Pol II through the nucleosome is unaffected by the acetylation or Nap1. These results indicate that H3K56 acetylation facilitates Pol II translocation through the nucleosome by assisting paused Pol II to resume and that Nap1 does not affect Pol II progression. Following transcription, only a small fraction of nucleosomes remain intact, which is unaffected by H3K56 acetylation or Nap1. These results suggest that (i) spontaneous nucleosome opening enables Pol II progression, (ii) Pol II mediates nucleosome reassembly very inefficiently, and (iii) Nap1 in the absence of other factors does not promote nucleosome disassembly or reassembly during transcription.
中文翻译:

转录延伸过程中的核小体动力学。
核小体是真核基因组的基本包装单位。DNA与核小体中组蛋白之间的动态相互作用是基因可及性调控的分子基础,可调控性控制着各种以DNA为模板的过程的动力学,例如RNA聚合酶II(Pol II)的转录延伸。基于化学修饰的组蛋白的单分子FRET测量,我们研究了转录延伸过程中的核小体动力学及其在H3 K56和组蛋白伴侣Nap1上受组蛋白乙酰化作用的影响,这两者均可影响DNA与组蛋白的相互作用。我们观察到H3K56乙酰化大大缩短了Pol II在核小体进入区域附近的停顿持续时间,而Nap1没有引起明显的差异。我们还发现,Pol II通过核小体的延伸率不受乙酰化或Nap1的影响。这些结果表明,H3K56乙酰化可通过协助暂停的Pol II恢复来促进Pol II通过核小体的转运,并且Nap1不会影响Pol II的进程。转录后,只有一小部分的核小体保持完整,不受H3K56乙酰化或Nap1的影响。这些结果表明(i)自发的核小体开放使Pol II进程得以实现,(ii)Pol II介导的核小体重组效率非常低,并且(iii)在没有其他因素的情况下,Nap1不会促进转录过程中核小体的分解或重组。这些结果表明,H3K56乙酰化可通过协助暂停的Pol II恢复来促进Pol II通过核小体的转运,并且Nap1不会影响Pol II的进程。转录后,只有一小部分的核小体保持完整,不受H3K56乙酰化或Nap1的影响。这些结果表明(i)自发的核小体开放使Pol II进程得以实现,(ii)Pol II介导的核小体重组效率非常低,并且(iii)在没有其他因素的情况下,Nap1不会促进转录过程中核小体的分解或重组。这些结果表明,H3K56乙酰化可通过协助暂停的Pol II恢复来促进Pol II通过核小体的转运,并且Nap1不会影响Pol II的进程。转录后,只有一小部分的核小体保持完整,不受H3K56乙酰化或Nap1的影响。这些结果表明(i)自发的核小体开放使Pol II进程得以实现,(ii)Pol II介导的核小体重组效率非常低,并且(iii)在没有其他因素的情况下,Nap1不会促进转录过程中核小体的分解或重组。
更新日期:2020-12-18
中文翻译:

转录延伸过程中的核小体动力学。
核小体是真核基因组的基本包装单位。DNA与核小体中组蛋白之间的动态相互作用是基因可及性调控的分子基础,可调控性控制着各种以DNA为模板的过程的动力学,例如RNA聚合酶II(Pol II)的转录延伸。基于化学修饰的组蛋白的单分子FRET测量,我们研究了转录延伸过程中的核小体动力学及其在H3 K56和组蛋白伴侣Nap1上受组蛋白乙酰化作用的影响,这两者均可影响DNA与组蛋白的相互作用。我们观察到H3K56乙酰化大大缩短了Pol II在核小体进入区域附近的停顿持续时间,而Nap1没有引起明显的差异。我们还发现,Pol II通过核小体的延伸率不受乙酰化或Nap1的影响。这些结果表明,H3K56乙酰化可通过协助暂停的Pol II恢复来促进Pol II通过核小体的转运,并且Nap1不会影响Pol II的进程。转录后,只有一小部分的核小体保持完整,不受H3K56乙酰化或Nap1的影响。这些结果表明(i)自发的核小体开放使Pol II进程得以实现,(ii)Pol II介导的核小体重组效率非常低,并且(iii)在没有其他因素的情况下,Nap1不会促进转录过程中核小体的分解或重组。这些结果表明,H3K56乙酰化可通过协助暂停的Pol II恢复来促进Pol II通过核小体的转运,并且Nap1不会影响Pol II的进程。转录后,只有一小部分的核小体保持完整,不受H3K56乙酰化或Nap1的影响。这些结果表明(i)自发的核小体开放使Pol II进程得以实现,(ii)Pol II介导的核小体重组效率非常低,并且(iii)在没有其他因素的情况下,Nap1不会促进转录过程中核小体的分解或重组。这些结果表明,H3K56乙酰化可通过协助暂停的Pol II恢复来促进Pol II通过核小体的转运,并且Nap1不会影响Pol II的进程。转录后,只有一小部分的核小体保持完整,不受H3K56乙酰化或Nap1的影响。这些结果表明(i)自发的核小体开放使Pol II进程得以实现,(ii)Pol II介导的核小体重组效率非常低,并且(iii)在没有其他因素的情况下,Nap1不会促进转录过程中核小体的分解或重组。











































 京公网安备 11010802027423号
京公网安备 11010802027423号