当前位置:
X-MOL 学术
›
Mol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Benzodiazepine Ligand with Improved GABAAReceptorα5-Subunit Selectivity Driven by Interactions with Loop C
Molecular Pharmacology ( IF 3.2 ) Pub Date : 2021-01-01 , DOI: 10.1124/molpharm.120.000067 Xenia Simeone 1 , Filip Koniuszewski 1 , Markus Müllegger 1 , Andreas Smetka 1 , Friederike Steudle 1 , Roshan Puthenkalam 1 , Margot Ernst 2 , Petra Scholze 2
Molecular Pharmacology ( IF 3.2 ) Pub Date : 2021-01-01 , DOI: 10.1124/molpharm.120.000067 Xenia Simeone 1 , Filip Koniuszewski 1 , Markus Müllegger 1 , Andreas Smetka 1 , Friederike Steudle 1 , Roshan Puthenkalam 1 , Margot Ernst 2 , Petra Scholze 2
Affiliation
|
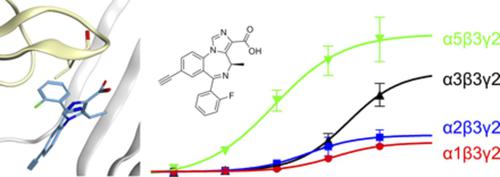
The family of GABAA receptors is an important drug target group in the treatment of sleep disorders, anxiety, epileptic seizures, and many others. The most frequent GABAA receptor subtype is composed of two α-, two β-, and one γ2-subunit, whereas the nature of the α-subunit critically determines the properties of the benzodiazepine binding site of those receptors. Nearly all of the clinically relevant drugs target all GABAA receptor subtypes equally. In the past years, however, drug development research has focused on studying α5-containing GABAA receptors. Beyond the central nervous system, α5-containing GABAA receptors in airway smooth muscles are considered as an emerging target for bronchial asthma. Here, we investigated a novel compound derived from the previously described imidazobenzodiazepine SH-053-2′F-R-CH3 (SH53d-ester). Although SH53d-ester is only moderately selective for α5-subunit–containing GABAA receptors, the derivative SH53d-acid shows superior (>40-fold) affinity selectivity and is a positive modulator. Using two-electrode voltage clamp electrophysiology in Xenopus laevis oocytes and radioligand displacement assays with human embryonic kidney 293 cells, we demonstrated that an acid group as substituent on the imidazobenzodiazepine scaffold leads to large improvements of functional and binding selectivity for α5β3γ2 over other αxβ3γ2 GABAA receptors. Atom level structural studies provide hypotheses for the improved affinity to this receptor subtype. Mutational analysis confirmed the hypotheses, indicating that loop C of the GABAA receptor α-subunit is the dominant molecular determinant of drug selectivity. Thus, we characterize a promising novel α5-subunit–selective drug candidate.
中文翻译:

一种苯二氮卓配体,通过与环 C 的相互作用,具有改进的 GABAAReceptorα5-亚基选择性
GABA A受体家族是治疗睡眠障碍、焦虑、癫痫发作和许多其他疾病的重要药物靶组。最常见的 GABA A受体亚型由两个α -、两个β - 和一个γ 2-亚基组成,而α-亚基的性质决定了这些受体的苯二氮卓结合位点的性质。几乎所有临床相关药物均同等地靶向所有 GABA A受体亚型。然而,在过去几年中,药物开发研究集中在研究含α 5 的 GABA A受体。在中枢神经系统之外,α气道平滑肌中含有 5 的 GABA A受体被认为是支气管哮喘的新兴靶点。在这里,我们研究了一种源自先前描述的咪唑苯并二氮杂卓 SH-053-2'FR-CH3(SH53d-酯)的新型化合物。虽然 SH53d-酯对含α 5 亚基的 GABA A受体仅具有中等选择性,但衍生物 SH53d-酸显示出优异的(>40 倍)亲和选择性,并且是一种正调节剂。在非洲爪蟾中使用两电极电压钳电生理学用人类胚胎肾 293 细胞进行的卵母细胞和放射性配体置换测定,我们证明了作为咪唑并苯二氮卓支架上的取代基的酸基团与其他α x β 3 γ 2 GABA A相比,导致α 5 β 3 γ 2 的功能和结合选择性大幅提高受体。原子水平结构研究为提高对这种受体亚型的亲和力提供了假设。突变分析证实了这些假设,表明 GABA A受体α亚基的环 C 是药物选择性的主要分子决定因素。因此,我们描述了一种有前途的新型α5-亚基选择性候选药物。
更新日期:2020-12-08
中文翻译:

一种苯二氮卓配体,通过与环 C 的相互作用,具有改进的 GABAAReceptorα5-亚基选择性
GABA A受体家族是治疗睡眠障碍、焦虑、癫痫发作和许多其他疾病的重要药物靶组。最常见的 GABA A受体亚型由两个α -、两个β - 和一个γ 2-亚基组成,而α-亚基的性质决定了这些受体的苯二氮卓结合位点的性质。几乎所有临床相关药物均同等地靶向所有 GABA A受体亚型。然而,在过去几年中,药物开发研究集中在研究含α 5 的 GABA A受体。在中枢神经系统之外,α气道平滑肌中含有 5 的 GABA A受体被认为是支气管哮喘的新兴靶点。在这里,我们研究了一种源自先前描述的咪唑苯并二氮杂卓 SH-053-2'FR-CH3(SH53d-酯)的新型化合物。虽然 SH53d-酯对含α 5 亚基的 GABA A受体仅具有中等选择性,但衍生物 SH53d-酸显示出优异的(>40 倍)亲和选择性,并且是一种正调节剂。在非洲爪蟾中使用两电极电压钳电生理学用人类胚胎肾 293 细胞进行的卵母细胞和放射性配体置换测定,我们证明了作为咪唑并苯二氮卓支架上的取代基的酸基团与其他α x β 3 γ 2 GABA A相比,导致α 5 β 3 γ 2 的功能和结合选择性大幅提高受体。原子水平结构研究为提高对这种受体亚型的亲和力提供了假设。突变分析证实了这些假设,表明 GABA A受体α亚基的环 C 是药物选择性的主要分子决定因素。因此,我们描述了一种有前途的新型α5-亚基选择性候选药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号