Synthesis ( IF 2.2 ) Pub Date : 2020-12-02 , DOI: 10.1055/s-0040-1706103 Narendra R. Chaubey 1 , Anant R. Kapdi 1 , Biswanath Maity 2
|
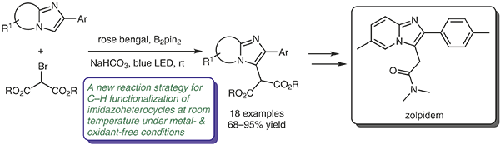
Organophotocatalytic C–H bond functionalization has attracted a lot of attention in the past several years due to the possibility of catalyzing reactions in a metal- and peroxide-free environment. Continuing on these lines, an organophotoredox-catalyzed C–H functionalization of imidazo[1,2-a]pyridines and related heterocycles with bromomalonates under mild conditions is reported, providing excellent yields of the products at room temperature. This is the first report involving malonates as coupling partners leading to the synthesis of a range of functionalized products including total synthesis of zolpidem, a sedative-hypnotic drug molecule.
中文翻译:

有机光氧化还原催化丙二酸酯与咪唑杂环的CH烷基化:唑吡坦的全合成
在过去的几年中,由于在无金属和无过氧化物的环境中催化反应的可能性,有机光催化的C–H键功能化引起了很多关注。沿这些路线继续,报道了在温和的条件下用溴代丙二酸酯对咪唑并[1,2- a ]吡啶和相关杂环进行有机光氧化还原催化的CHH功能,可在室温下提供优异的收率。这是第一份涉及丙二酸酯作为偶联伙伴的报告,导致合成了一系列功能化产品,包括镇静催眠药物分子唑吡坦的全合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号